Abstract
Background
Task shifting has become an increasingly popular way to increase access to health services, especially in low-resource settings. Research has demonstrated that task shifting, including the use of community health workers (CHWs) to deliver care, can improve population health. This systematic review investigates whether task shifting in low-income and middle-income countries (LMICs) results in efficiency improvements by achieving cost savings.
Methods
Using the PRISMA guidelines for systematic reviews, we searched PubMed, Embase, CINAHL, and the Health Economic Evaluation Database on March 22, 2016. We included any original peer-review articles that demonstrated cost impact of a task shifting program in an LMIC.
Results
We identified 794 articles, of which 34 were included in our study. We found that substantial evidence exists for achieving cost savings and efficiency improvements from task shifting activities related to tuberculosis and HIV/AIDS, and additional evidence exists for the potential to achieve cost savings from activities related to malaria, NCDs, NTDs, childhood illness, and other disease areas, especially at the primary health care and community levels.
Conclusions
Task shifting presents a viable option for health system cost savings in LMICs. Going forward, program planners should carefully consider whether task shifting can improve population health and health systems efficiency in their countries, and researchers should investigate whether task shifting can also achieve cost savings for activities related to emerging global health priorities and health systems strengthening activities such as supply chain management or monitoring and evaluation.
Keywords: Task shifting, Community health workers, Health systems, Efficiency, Cost-effectiveness, Systematic review
Background
Efficient and effective health systems are critical for managing healthcare costs, addressing rising burden of disease, and providing sustainably universal health coverage. The efficiency of health spending has major implications for the health of the population. In low-income and middle-income countries (LMICs) of Africa, Asia, and the Middle East, increasing the efficiency of health spending could increase health-adjusted life expectancy by 1–2 years [1].
Human resources for health (HRH) make up a significant portion of health expenditures; in LMICs, spending on salaried health workers makes up 28.7–33.2% of total health expenditure [2]. Improving the efficiency of spending on HRH can improve the efficiency of health systems, which can free up financial and other resources and ultimately improve health coverage [3].
According to the World Health Organization (WHO), task shifting “presents a viable solution for improving health care coverage by making more efficient use of the human resources already available and by quickly increasing capacity while training and retention programs are expanded” [4]. Task shifting can produce equivalent or superior outcomes for many diseases and health interventions including non-communicable diseases [5], HIV/AIDS [6, 7], contraceptive distribution [8], and others [5, 9].
Given the high spend on HRH, the evidence for task shifting as a way to improve population health, and the prominence of task shifting on the global policy agenda, policymakers should understand the cost and efficiency implications of this approach to health systems strengthening (HSS). Therefore, our systematic review aims to answer the following question: Does task shifting result in cost savings and efficiency improvements for health systems or patients in LMICs?
To our knowledge, only one literature review has addressed a similar question so far [10]. That review found that community health workers (CHWs) are cost-effective for treating TB and select other disease areas, such as reproductive, maternal, newborn, and child health (RMNCH). Our review builds on the important initial review conducted by Vaughan et al. in three ways. First, our search strategy takes a broader scope in that it reviews other forms of task shifting besides the use of CHWs (e.g., shifting the work of physicians to nurses or the work of nurses to pharmacy technicians), which may contribute to HSS.
Second, our review looks at evidence for efficiency improvements achieved by shifting tasks from one cadre of workers to another, rather than whether an intervention using a specific type of health worker meets a cost-effectiveness threshold. Although cost-effectiveness thresholds (e.g., cost/unit of health improvement above or below a pre-defined benchmark) are an important criterion for prioritizing interventions, cost-effectiveness as measured by an actual reduction in costs without a reduction in programmatic quality is particularly salient for policymakers trying to improve the efficiency of the health system. Therefore, we review whether studies found changes in cost per input/process, output, or outcome as a result of task shifting. Whereas cost savings on inputs/processes are very likely since the wage for a lower-skilled worker will almost always be lower than that of a higher-skilled worker, cost savings on outputs and outcomes are not as guaranteed since lower-skilled workers might operate less efficiently. A reduction in cost per output or outcome can be interpreted as an improvement in efficiency and therefore a true savings to the health system (with changes in cost per outcome as the stronger indicator), but a reduction in cost per input/process can only be interpreted as an efficiency improvement if it is accompanied by the documentation of no change (or an improvement) in clinical or programmatic quality.
Third, following from the previous point, our review also captures and reports evidence of changes in programmatic or clinical quality as a result of task shifting for each included reference, which Vaughan et al. do not systematically report. Reporting programmatic quality outcomes is important for determining whether a reduction in costs actually indicates an improvement in health systems efficiency.
Methods
This systematic review follows the criteria and methodology described in the PRISMA guidelines on systematic reviews [11].
Search process and criteria
This search relied on an internal protocol developed by both authors, with the support of a Harvard University librarian specializing in systematic reviews. The protocol was not registered externally. We searched PubMed, Embase, CINAHL, and the Health Economic Evaluation Database. The main search that was conducted on March 22, 2016, was as follows (for PubMed), with an additional search term for LMICs, and any publication from before that data was eligible for our review:
(task shift*[tiab] OR balance of care[tiab] OR non-physician clinician*[tiab] OR nonphysician clinician*[tiab] OR task sharing[tiab] OR community care giver*[tiab] OR community healthcare provider*[tiab] OR cadres[tiab] OR “Community Health Workers”[Mesh])
AND
(“Cost Savings”[mesh] OR “Cost Benefit Analysis”[mesh] OR “Efficiency”[mesh] OR cost[tiab] OR costs[tiab] OR efficienc*[tiab] OR economies of scale[tiab] OR economies of scope[tiab] OR productivity[tiab] OR absenteeism[tiab] OR “Absenteeism”[Mesh])
We also conducted several additional searches based on a review of citation lists from relevant publications, and based on recommendations from public health researchers.
Study selection and eligibility criteria
After conducting our search, all titles were reviewed for relevance. After excluding irrelevant titles, we read all abstracts and, when appropriate, full articles to determine the relevance of the article for our research question. In order to be included in the study, the publication had to meet the following criteria:
Report on an effort, such as a program or policy intervention, involving task shifting of a clinical activity or health systems-related activity
Report a comparison of program costs from the task shifted model for conducting the activity or service to a comparable activity in a model that does not involve task shifting.
Report results from an actual intervention, rather than a computer model or simulation
Report results from a low-income or middle-income country
Be original research about an intervention published in a peer-reviewed format (as opposed to an editorial, literature review, opinion piece, interview, etc.)
Have a complete article available (as opposed to just an abstract)
Be published in English
Data collection process
In order to extract data for this review, we piloted an Excel-based data collection tool that was used to capture results from a preliminary search, the results of which were presented at the Harvard Ministerial Leadership Program in the summer of 2016. Based on our experience with this initial process, we modified the tool accordingly and finalized a tool which collected the following information: author, year, title, publication, abstract, country, continent, description of the intervention, main indicator, result on relevant indicator, and data on programmatic quality changes resulting from the intervention. Studies were not excluded if they did not have relevant quality comparisons. Results which did not provide evidence of cost changes, such as baseline costing studies, were excluded. GS conducted a first review of all references in the search, and the list was reviewed by RA and other public health researchers in order to identify missing references or references which had been improperly included.
We also retrospectively categorized the included references based on whether the main indicator documented changes in cost per input/process, output, or outcome, using the following definitions: [12].
Inputs/processes: resources required to conduct an activity, or a discrete activity such as a patient visit with a clinician
Outputs: direct products of program activities, such as number of individuals treated
Outcomes: changes in health status as a result of the program, such as number of patients cured or number of deaths averted
Risk of bias
As with any systematic review, the references and data sources for this review contain the possibility for bias. At the level of individual references, authors are more likely to report cost data if their program resulted in cost savings, especially if costing/cost-effectiveness was not the primary purpose of the study.
Across all studies, there is also a risk of publication bias and selective reporting within studies, especially if authors more frequently chose to report positive outcomes (such as cost savings). Of course, the decision to implement task shifting in a given context would require extensive analysis of that particular intervention’s potential impact, and we caution researchers and policymakers not to interpret the findings from this review as indicative of the results that they can expect to achieve.
Results
Study selection
We reviewed 791 articles and identified 34 references which analyzed the cost implications of task shifting in LMICs—22 in sub-Saharan Africa, eight in Asia and four in Central or South America. See Fig. 1 for the study selection for inclusion in this systematic review. Of the 32 studies included in the review by Vaughan et al., we excluded 17 and included 15, which means that our review also included an additional 19 studies not included in Vaughan et al. Of the 17 references included by Vaughan et al. that we excluded, 12 were excluded because they did not provide comparison of costs between the task shifted model and another model of care [13–24], three reported results from modeling of hypothetical programs rather than actual interventions [25–27], one reference did not have a full article available [28], and one reference reported the same data from the same program as another reference already included in our review [29].
Fig. 1.
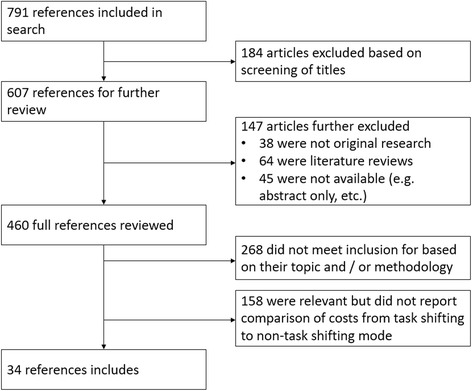
Study selection for inclusion in systematic review
Of the 34 studies included in our review, 30 found evidence of a reduction in health costs either to the health system or the patient, and four had a mixed impact, an increase in costs, or no changes in costs [30–33]. Almost all the studies focused on the effects of shifting clinical or public health tasks related to a specific disease or disease area, while one study focused on task shifting a HSS activity (mapping of village geographic coordinates) [34]. Only two studies examined task shifting within a hospital, whereas all others examined task shifting from the hospital to the primary health care (PHC) or community levels, or task shifting within the PHC/community level.
Of the 30 studies that found evidence of cost savings, 10 reported a cost savings per outcome, 13 reported a cost savings per output, and 3 reported a cost savings per input/process coupled with a corresponding maintenance or improvement in programmatic quality. Although cost savings on inputs/processes do not indicate efficiency improvements as strongly as savings on outputs or outcomes, the combined body of evidence from these 26 studies suggests that task shifting yields cost savings that result in efficiency improvements to the health system, especially at the PHC and community levels. The four citations which reported cost savings on an input/process and which did not report changes in clinical or programmatic quality all reported on tasks related to different disease areas/HSS activities.
The full list of references meeting inclusion criteria can be found in Table 1.
Table 1.
Full list of citations included in systematic review
| Author and year | Country | Intervention | Indicator type | Main indicator | Result | Quality data |
|---|---|---|---|---|---|---|
| TB | ||||||
| Clarke, M., et al. (2006) [39] | South Africa | Training of lay health workers (LHWs) to support treatment and management of TB on farms, instead of clinic nurses or enrolled (non-professional) nurses | Input/process | Cost per minute of health worker time | 91% reduction in cost from clinic nurses ($0.12 per minute) to LHWs ($0.01 per minute) and 87.5% reduction from enrolled nurses ($0.08 per minute) to LHWs | Farms with LHWs supporting had 42% better case finding rate and 10% better cure rate |
| Datiko, D. G. and B. Lindtjorn (2010) [35] | Ethiopia | Comparison of Health Facility-based DOT (HFDOT) program for TB compared with community DOT (CDOT) program using health extension workers | Outcome | Cost per successfully treated patient | 63% reduction in costs from HFDOT model ($16.19) to CDOT model ($6.07) | 74.8% cure rate for CDOT compared with 68.2% for HFDOT |
| Dick, J., et al. (2007) [37] | South Africa | Evaluation of a lay health worker project overseen by primary healthcare nurses aimed at treating TB on farms | Outcome | Cost per case detected and cured | 74% cost reduction to the District Health Authority on farms with LHW program compared to control farms (absolute cost figures not reported) | Treatment completion rate for smear-positive TB patients 18.7% higher in intervention group compared to controls (p < .05) |
| Floyd, K., et al. (2003) [41] | Malawi | Community-based outpatient treatment for smear-positive pulmonary patients (instead of inpatient treatment) | Outcome | Cost per patient cured | 62% reduction from hospital-based treatment ($786) to community-based treatment ($296) | Cure rate was 68% for community-based strategy and 58% for hospital-based strategy |
| Islam, M. A., et al. (2002) [36] | Bangladesh | BRAC TB control program using CHWs, compared to government-run program | Input/process; outcome | Total annual cost for TB control program at the subdistrict (thana) level; Cost per patient cured | 31% reduction in total annual costs from government program ($10,697) to BRAC program ($7,351); 32% reduction in cost per patient cured | 84.1% cure rate in BRAC TB program compared to 82.2% in government program |
| Khan, M. A., et al. (2002) [40] | Pakistan | Comparison of DOTS by health workers at health centers, DOTS by family members, and “DOTS without direct observation” | Outcome | Cost per case cured | 45% reduction from health center DOTS ($310) to CHW DOTS ($172); unsupervised DOTS cost $164 | Cure rates were 62% for unsupervised DOTS, 55% for family member DOTS, 67% for CHW DOTS, and 58% for Health Center DOTS |
| Okello, D., et al. (2003) [38] | Uganda | Comparison of conventional hospital-based care with community-based care for DOTS, including management by a sub-county public health worker | Outcome | Cost per smear-positive patient successfully treated | 57% reduction in costs from conventional care ($911) to community-based care ($391) | Treatment success rate for smear-positive cases was 56% for conventional care and 74% within community-based care |
| Prado, T. N., et al. (2011) [42] | Brazil | Comparison of DOTS overseen by guardians with standard of care treatment by CHWs | Output | Total cost for DOTS course | 28% reduction in costs from CHW DOTS ($547) to guardian-supervised DOTS ($389) | 98% treatment completion in guardian-supervised DOTS compared to 83% treatment completion with CHW-supervised DOTS (p = .01) |
| Sinanovic, E., et al. (2003) [43] | South Africa | Comparison of clinic-based care with community-based observation by lay person with community-based care for smear-positive pulmonary and retreatment TB patients | Outcome | Cost per patient successfully treated | 62% reduction in costs for new smear-positive patients from clinic-based care ($1302) to community-based care ($392); 62% reduction in costs for retreatment patients from clinic-based care ($2008) to community-based care ($766) | 80% treatment success rate for community-based care, compared to 54% treatment success rate for clinic-based care |
| HIV | ||||||
| Babigumira, J. B., et al. (2011) [46] | Uganda | Comparison of a Pharmacy-only Refill Program (PRP) to Standard of Care for treatment for HIV/AIDS patients | Output | Cost per person per year from societal and Ministry of Health perspective | 21% reduction in societal costs from Standard of Care ($665) to PRP ($520) and 17% reduction in MoH costs from Standard of Care ($610) to PRP ($496) | No statistically significant difference in favorable immune response among patients in two groups |
| Bemelmans, M., et al. (2014) [48] | South Africa | Adherence club for ARVs led by lay counselor and offered to all clinically stable patients who had been on ARVs for greater than 12 months; Club met every 2 months for essential medical tasks (e.g., weighing and health assessment) and distribution of ARVs | Output | Cost per patient per year | 46% reduction from mainstream model of care ($108) to ARV club model ($58) | <1% mortality at 40 months, and 2.8% loss to follow up at 40 months in ARV club |
| Fatti, G., et al. (2015) [45] | South Africa | Indirectly Supervised Pharmacist Assistant (ISPA) program compared to nurse-managed models for providing ARTs | Input/process | Human resource costs and costs per item dispensed | 29% reduction in human resource costs from nurse-managed program ($1.89 per patient visit) compared to ISPA model ($1.35 per patient visit); 49% reduction in cost per item dispensed from nurse-managed program ($0.83) to ISPA model ($0.43) | Cumulative attrition lower at ISPA sites (20.7% compared to 31.5%); proportion of patients achieving virological suppression higher at ISPA sites (89.6% compared to 85.9%) |
| Foster, N. and D. McIntyre (2012) [47] | South Africa | Indirectly Supervised Pharmacist Assistant (ISPA) program and nurse-managed models compared to full-time pharmacist for providing ARTs | Input/process | Cost per patient visit | 43% reduction in cost from nurse-driven model ($10.16) to ISPA model ($5.74) and 12% reduction in cost from full-time pharmacist model ($6.55) | |
| Johns, B. and E. Baruwa (2015) [31] | Nigeria | Comparison of hospital-based distribution of ART (by doctors) with clinic-based distribution of ART (by nurses and/or community pharmacists) for stable patients who had been on ART for at least 1 year, in two states aiming to decentralize health services | Output | Total cost per person per year | Total costs increased in one state by 31% and decreased in one state by 32%; In both cases, the largest difference in costs between the hospital and clinic sites was staff cost/patient visit | Few statistically significant differences found in service utilization indicators between patients going to clinic sites versus hospital sites; Patients in the state that achieved cost savings had 3.7× more visits per year than in hospitals (p < .01) |
| Johns, B., et al. (2014) [30] | Ethiopia | Comparison of minimal, moderate, and maximal task shifting for ARV responsibilities away from physicians with hospital-based ARV distribution . Minimal = nonphysicians clinicians (NPC) monitor ART; Moderate = NPC initiate and monitor ART; Maximal = NPCs initiate, monitor, treat side effects, and switch ARTs | Output | Cost per patient year | No statistically significant changes in cost/patient per year between models of task shifting or between all task shifting models and hospitals | Almost no statistically significant differences in patient retention from different levels of task shifting |
| Yan, H., et al. (2014) [44] | China | Evaluation of shifting HIV preventive intervention and care for men who have sex with men (MSM) from government facilities to community-based organizations (CBOs) | Outcome | Unit cost per HIV case detected | 97% reduction in cost from government health facilities ($14,906) to community-based organizations ($315) | Within 4 years, total % of HIV cases reported increased from ~10 to ~50%, despite “a very low share of HIV tests by CBOs out of the total HIV tests performed each year during the pilot,” which indicates effective targeting of HIV patients for tests by CBOs |
| Malaria | ||||||
| Chanda, P., et al. (2011) [49] | Zambia | Comparison of home management (using CHW) with facility-based management of uncomplicated malaria | Output | Cost per case appropriately diagnosed and treated | 31% reduction from facility-based management ($6.12) to home management ($4.22) | 100% of cases treated appropriately through home management, and 43% of cases treated appropriately in facility |
| Hamainza, B. M., et al. (2014) [50] | Zambia | Comparison of CHW program to test and treat malaria with facility-based testing and treatment | Output | Total cost per confirmed case treated | 60% reduction in cost from facility-based approach ($10.75) to CHW approach ($4.34) | 78% of CHW contacts received appropriate testing and treatment, while 53% of facility-based patients received appropriate testing and treatment based on guidelines |
| Mbonye, A., et al. (2008) [32] | Uganda | Community-based administration of intermittent preventive treatment (IPTp) for malaria by traditional birth attendants, drug-shop vendors, community reproductive health workers, and adolescent peer mobilizers | Output | Cost per patient of providing a full regimen of IPTp | 9% increase in costs from health center care (4093 shillings) to community-based care (4491 shillings) | |
| Patouillard, E., et al. (2011) [51] | Ghana | Comparison of IPT administration by village health workers (VHWs), facility-based nurses working in outpatient departments of health centers or EPI outreach clinics | Outcome | Economic cost per child fully covered and fully adherent to treatment | 11% reduction from using facility-based strategy ($8.51) to VHW strategy ($7.56) | 69.1% of children in VHW strategy completed course, 63.8% of children in facility-based strategy completed course |
| Ruebush, T. K., 2nd, et al. (1994) [52] | Guatemala | Change to the supervision and distribution model of unpaid Volunteer Collaborators (VC) in the surveillance and treatment of malaria, including treatment for malaria without taking a blood smear, removal of literacy requirement for VC, and reduced supervision from once every 4 weeks to once every 8 weeks | Output | Cost per patient treated | 75% reduction in cost per patient treated in modified model of VCs ($0.61) versus control network of VCs ($2.45) | Average time from examination to initiation of treatment was 6.6 days in modified model areas, compared to 14.6 days in control areas |
| Sikaala, C. H., et al. (2014) [53] | Zambia | Community-based (CB) mosquito surveillance and trapping using light traps (LT) and Ifakara tent traps (ITT) compared to centrally supervised quality assurance (QA) trapping teams, including human-landing catch (HLC) teams, for the prevention of malaria | Output | Cost per specimen of Anopheles funestus captured | 96% reduction in costs from using QA-LT ($141) to CB-LT ($5.3); 83% reduction in costs from using QA-ITT ($168) to CB-ITT ($28); QA-HLC method cost $10.5 | |
| Other diseases and health systems strengthening activities | ||||||
| Aung, T., et al. (2013) [62] | Myanmar | Comparison of costs to treat diarrhea by CHW, government facility, and private provider | Input/process | Total patient cost for consultation and correct ORS | 7% reduction from private providers ($5.40) to CHWs ($5) and 67% reduction from government facilities ($15) to CHWs | CHWs provided appropriate ORS and amount of drinking water in 57.6% of cases, private providers in 47.1% of cases, and government facilities in 71.4% of cases |
| Buttorff, C., et al. (2012) [57] | India | Comparison of “collaborative care” model using full-time physician, lay health worker (LHW), and mental health specialist with “enhanced usual care” by full-time physician only for treatment of depression and anxiety disorders | Output | Average annual cost per subject | 23% reduction in costs from collaborative care model ($177) compared to physician-only care model ($229) | Patients in collaborative care improved 3.84 points more on the Revised Clinical Interview Schedule (to measure psychiatric symptoms) compared to physician-only care model |
| Chuit, R., et al. (1992) [60] | Argentina | Surveillance to reduce transmission of Chagas disease using Primary Health Care (PHC) agents compared to a vertically oriented program run by trained entomological professionals | Output | Cost of surveillance per house | 80% reduction in cost from vertical surveillance ($17) to PHC surveillance ($3.40) | Surveillance rates and levels of infestation detection were comparable across intervention and control arms |
| Cline, B. L. and B. S. Hewlett (1996) [61] | Cameroon | Diagnosis and treatment for schistosomiasis by CHWs identified by the community | Output | Average cost of diagnosis and treatment of a child | 90% reduction in cost from treatment at nearest pharmacy (approx. $15) to CHW model ($1.50) | 7% prevalence in school children after participating in program, compared to 71% in children who did not participate in program |
| Fiedler, J. L., et al. (2008) [63] | Honduras | Community-based integrated child care (AIN-C) program that uses volunteers to help mothers monitor and maintain adequate growth of young children | Input/process | Cost for one child growth and development consultation | 86% reduction from facility-based consultation (105.1 lempiras) to community-based program (14.67 lempiras) | |
| Hounton et al., (2009) [33] | Burkina Faso | Training of obstetricians, general practitioners, and clinical officers to lead surgical teams for caesarian sections | Outcome | Incremental cost of one newborn life saved | Compared to clinical officers, one newborn life saved cost $200 for general practitioners, and $3,235 for obstetricians | Higher newborn and maternal case fatality rates among clinical officers than other types of practitioners |
| Jafar, T. H., et al. (2011) [54] | Pakistan | Home-health education (HHE) by CHWs, home-health education plus general practitioner (GP) supervision (combined group), or general practitioner-supervision only to control blood pressure | Output | Total cost per patient over 2 years for each group | 7% reduction in costs from GP-only group ($537) to combined group ($500); 27% reduction in costs from GP-only group to HHE-only group ($393) | Decline in systolic BP was highest in the combined group (p = .001) |
| Kruk, M. E., et al. (2007) [58] | Mozambique | Comparison of surgically trained assistant medical officers and specialist physicians | Input/process | Cost per major obstetric surgical procedure | 72% reduction in costs using assistant medical officers ($39) compared to specialist physicians ($144) | |
| Laveissiere, C., et al. (1998) [56] | Cote d'Ivoire | Detection of sleeping sickness using conventional mobile teams compared to integration of activity into CHW duties | Output | Cost of surveillance per person | 81% reduction in costs using CHWs ($0.10) instead of using mobile teams ($0.55) | |
| Puett, C., et al. (2013) [55] | Bangladesh | Community-based management of severe acute malnutrition by CHWs compared to inpatient treatment | Outcome | Cost per DALY averted | 98% reduction in costs/DALY averted from observed inpatient treatment costs ($1344) to community treatment ($26) and in costs/death averted from observed inpatient treatment costs ($45,688) to community treatment ($869) | 91.9% of children in community treatment area recovered, compared to only 1.4% in inpatient treatment |
| Sadruddin, S., et al. (2012) [59] | Pakistan | Comparison of home treatment of severe pneumonia by lady health workers with referred cases treated by other practitioners | Output | Cost per treatment of severe pneumonia | 81% reduction in costs using lady health workers ($1.46) compared to referred cases ($7.60) | 93.4% of cases successfully treated by lady health workers with a 5-day course of amoxicillin, and remaining cases referred for further treatment |
| Munyaneza, F., et al. (2014) [34] | Rwanda | Use of CHWs and nurses to collect geographic coordinates using GIS systems instead of trained and dedicated GIS teams | Input/process | Total cost of mapping activities | 51% reduction in costs from using dedicated GIS teams ($60,112) to CHWs ($29,692) | |
Tuberculosis
Nine studies demonstrated cost savings with task shifting for identification, diagnosis, and treatment of tuberculosis. Strategies for reducing costs included task shifting treatment supervision to health workers in the community [35–41], to home guardians or close relatives [42], laypersons [43], and in one case entrusting patients to take medicine without direct supervision [13]. Programmatic and clinical indicators, such as treatment success rate, treatment completion rate, and case finding rate, also indicate that task shifting programs maintained programmatic quality comparable or superior to traditional models of care.
HIV/AIDS
Studies in this review revealed cost savings from task shifting prevention and care for a high-risk group (men who have sex with men (MSM)) to community-based organizations [44], and dispensing of ART from pharmacists to Indirectly Supervised Pharmacist Assistants (ISPA), adherence clubs, or other pharmacy-only refill programs [45–48]. Programmatic indicators, such as patient retention, viral load, and mortality also indicate that these programs maintained high quality of care. These findings indicate that the dispensation of ARTs, especially to clinically stable patients who are very familiar with the routine of taking these drugs, is suitable for task shifting in low-resource (and possibly other) settings. One study examining task shifting of ART dispensation to clinics found both an increase of costs in one state and a decrease in another state [31], and one study examining the task shifting initiation and management of ART treatment found no statistically significant differences in costs [30].
Malaria
Our review identified five articles that identified cost savings related to task shifting for malaria-related programs: CHW management of malaria [49, 50], village health worker (VHW) administration of IPT [51], community-based surveillance and treatment of malaria [52], and community-based surveillance and trapping of mosquitoes for vector control [53]. Indicators of program and clinical quality, such as administration of appropriate treatment, treatment completion rate, and average time from examination to initiation of treatment, indicate that the programs also maintained or improved programmatic quality. One study found a minor (9%) increase in the cost of administration of IPT during pregnancy when shifting to a community-based model. Although the evidence is less robust than that for TB or HIV/AIDS, these findings suggest that many malaria-related tasks can achieve cost savings from task shifting.
Other disease areas and activities
Our review identified 11 additional studies which provided evidence of cost savings from task shifting for activities related to other diseases or health systems strengthening. These activities included controlling blood pressure through a combination of general practitioner and CHW activities [54], community-based management of severe acute malnutrition [55], integration of the detection of sleeping sickness intro routine CHW activities [56], treatment for mental health problems by a “collaborative care” team that included a lay health worker and mental health specialist [57], administration of major obstetric procedures by assistant medical officers instead of physicians [58], home-based treatment of severe pneumonia by lady health workers [59], integration of surveillance to reduce transmission of Chagas disease by Primary Health Care agents instead of specially trained professionals [60], diagnosis and treatment of schistosomiasis by CHWs [61], treatment of diarrhea by CHWs [62], community-based integrated child care using volunteers to monitor and maintain growth [63], and geo-mapping activities by CHWs and nurses instead of dedicated GIS teams [34].
Discussion
This review aimed to identify whether task shifting can result in cost savings and efficiency improvements to health systems. Our results indicate that task shifting is a promising approach to achieving cost savings and improving efficiency in LMICs, and our results build on previous work which concluded that task shifting can be an effective way to improve population health. These findings have significant policy implications, discussed below, as well as important limitations.
-
Task shifting can help achieve cost savings and improve efficiency for activities related to top global health priorities, emerging global health issues, and neglected tropical diseases, but the evidence base is mostly limited to PHC and community-based care
The most robust body of evidence found in this study is for achieving cost savings from task shifting activities related to TB and HIV/AIDS. Given the high burden of these diseases in LMICs and the longitudinal nature of preventing, treating, and managing these diseases, interventions that can reduce both their economic and health burdens simultaneously are particularly important for the future of global health. Each year there are 1.5 million new cases of tuberculosis, mostly in LMICs, and the global burden of TB amounts to approximately $12 billion annually [64, 65]. As of 2015, 36.7 million people were living with HIV, and meeting UNAIDS targets will require nearly $20 billion annually [66, 67]. TB treatment using DOTS is a relatively routine activity that occurs over many months and can take place in the community (when the infection is not drug-resistant). Dispensation of ART to clinically stable patients who know and follow their drug regimens is also a relatively routine process. Therefore, these activities are well-suited for task shifting, and health systems can likely improve their efficiency by undertaking such efforts.
Outside of TB, HIV/AIDS, and malaria, the evidence for cost savings from task shifting was spread across many disease areas, making it difficult to conclude that task shifting activities for a specific disease could result in cost savings. Nonetheless, the fact that programs achieved cost savings from such a diverse set of diseases and across multiple geographies indicates that policymakers and program planners should consider task shifting as one of many potential approaches to improve efficiency in their health systems. The evidence for cost savings came from disease areas such as childhood illnesses, non-communicable diseases (which are receiving increased priority at the global level due to the Sustainable Development Goals), and neglected tropical diseases (NTDs).
Almost all studies identified shifted tasks to or within the context primary health care (PHC) or community-based care. Although several citations identified cost savings by shifting tasks from hospitals to PHC or community care, only one citation found cost savings by shifting tasks within the hospital setting [58]. One additional study within the hospital setting found that shifting surgical care from physicians to clinical officers did not yield cost savings, but it did not analyze the cost-effectiveness of shifting surgical tasks from surgeons to other physicians [33]. While the body of evidence in this review suggests that task shifting can improve efficiency across multiple disease at the PHC and community levels, more research is needed on the effects of task shifting within secondary, tertiary, and highly specialized care.
-
Models of task shifting involve more than transferring clinical care to CHWs
CHWs play a key role in reducing costs and increasing access to care in the health system. Nonetheless, this research shows that many models of task shifting exist outside of a simple transfer of clinical care to a CHW. Of course, many types of associate health professionals exist, such as pharmacy technicians, lay counsellors, and medical assistants, and the references included in this study reflect this diversity of health professions [68]. In particular, the use of different models for dispensing ART to HIV-positive patients was documented in multiple studies. In addition, several studies used models where CHWs or other lower-skilled workers collaborated with clinicians in order to provide a new model of care for the patient [54, 57].
Interestingly, only two studies identified cost savings from task shifting non-clinical activities: geo-mapping by CHWs and community-based mosquito trapping and surveillance. Given the importance that many non-clinical health systems functions have on improving population health (e.g., supply chain, monitoring and evaluation), research and program planners should consider the potential that task shifting could have for other health systems-related activities. For example, it is possible that lower-skilled professionals could perform routine tasks related to monitoring the supply chain or tracking patient data without compromising the quality of the activity.
-
The design and benefits of task shifting interventions will vary based on the context
Policymakers and program planners must recognize that task shifting is not a panacea for improving health and efficiency, but rather one of many tools to use in order to improve the efficiency of the health system. This review identified a range of task shifting models which resulted in different types of cost savings. Of course, without proper design, task shifting may actually increase system costs or reduce efficiency, such as by worsening overall population health due to poor clinical quality or increasing the number of staff in the health care system without changing care-seeking patterns among patients. Interestingly, one study found that the same model of task shifting resulted in both cost increases and cost decreases in two different regions of the same country [31]. Further, task shifting can also result in task overload for health workers, which could also reduce productivity and worsen health population health outcomes [69].
The breadth of task shifting models covered in this review is consistent with other findings from the literature which also indicate the need to adapt task shifting models to local contexts and health systems. For example, one systematic review notes a number of factors which can impact the success of lay health worker programs, including acceptability of the model to patients, implementation challenges such as problems with training, and health systems bottlenecks such as challenges with payment [70]. Another systematic review specifically identified strong management of CHW programs as the most important factor in their scale-up [71]. This body of evidence therefore suggests that designing appropriate task shifting models requires a thorough investigation of the local context, disease burden, and program goals.
Limitations of the evidence, risks, and future directions for research
There are several limitations to the research and its findings. First, this study includes citations that measure changes in cost and efficiency very differently. Of course, looking strictly at cost-effectiveness thresholds, rather than cost savings and programmatic indicators as a proxy for cost-effectiveness, would have helped to standardize these findings to make them more comparable. However, limiting our analysis to cost-effectiveness thresholds would also have negatively altered the evidence base in our review by (1) eliminating studies which demonstrated savings but did not have a formal cost-effectiveness analysis and (2) including studies that may have achieved some level of cost-effectiveness but which did not actually achieve savings (i.e., those in which an intervention by a specific cadre of health worker met a cost-effectiveness threshold). By researching the impact of task shifting on costs to the health system as a proxy measure for efficiency improvements, we have focused on a key aspect of decision-making directly relevant to policymakers.
Second, unlike systematic reviews looking at health outcomes from highly specified clinical protocols, this review cannot predict the implications of a new task shifting program. Numerous factors in a given context will affect the outcomes of task shifting, including the burden of disease, the existing human resources for health, previous task shifting efforts, the social determinants of health, and the political economy of health. We caution that researchers and policymakers should not treat this review as a guarantee that future task shifting efforts will result in cost savings; rather, they should see this review as providing compelling evidence that task shifting can achieve cost savings if there is a need for such an intervention, and it is implemented appropriately.
Third, our search only identified two citations suitable for inclusion that examined task shifting within a hospital setting. Our search did not exclude programs that delivered services at a specific level, and the search included other citations focused on hospitals or specialty care that failed to meet inclusion criteria for other reasons (see select citations for examples [72–75]). This result suggests that the absence of evidence for task shifting within hospitals is likely due to the limited research on this topic to date. Nonetheless, LMICs have implemented programs to task shift hospital-based care, such as surgical services [76, 77]. Future research should examine models of task shifting within hospitals and their impacts on health outcomes, costs, and other relevant indicators.
Finally, as already discussed, the methodology of this review is limited by biases in reporting and publication of individual references.
Going forward, we feel that researchers, program planners, and policymakers should continue to collaborate to understand both the financial and health impacts of task shifting. Many new task shifting efforts are underway globally, and ensuring that all these programs report on cost-effectiveness thresholds and changes in costs to the system will increase the evidence base surrounding this important topic. In particular, more programmatic research is needed to confirm the preliminary findings that task shifting for activities related to NCDs, NTDs, and health systems strengthening can result in cost savings, and to understand the role that task shifting can play in hospital and specialty settings. At the same time, researchers should also carefully examine the risk of task overload from task shifting and design ways to prevent and mitigate this risk.
Conclusions
This review examined the evidence for task shifting in improving health systems efficiency in LMICs. The evidence indicates that task shifting for activities across a broad range of diseases, including TB, HIV/AIDS, malaria, childhood illness, NCDs, and NTDs, can result in cost savings without compromising clinical or programmatic quality. This review also revealed that countries have used different approaches to introduce task shifting for management of different conditions and that task shifting takes on many forms besides simply transferring clinical activities to CHWs. Going forward, researchers, program planners, and policymakers should carefully examine their local context in order to determine whether task shifting can improve health systems efficiency while also maintaining or improving population health.
Acknowledgements
We thank Michael Sinclair and Brian Dugan from the Harvard Ministerial Leadership Program for their support in preparation of this report. We thank Paul Bain at Harvard University for assistance with designing the search strategy.
Funding
An original draft of this paper was commissioned by the Harvard Ministerial Leadership Program, a joint initiative of the Harvard TH Chan School of Public Health, Harvard Kennedy School of Government, and the Harvard Graduate School of Education in collaboration with Big Win Philanthropy, and with the support of the Bill and Melinda Gates Foundation, Bloomberg Philanthropies, the GE Foundation, and the Rockefeller Foundation.
Availability of data and materials
Key information from original dataset included in Table 1 is in the manuscript. The original dataset is available from the corresponding author upon request.
Authors’ contributions
GS and RA jointly conceived of the research question, concept, and methodology for this paper. GS developed the data collection tool, reviewed all articles, and drafted and revised the manuscript. RA provided revisions and additional references for review. Both authors read and approved the final manuscript.
Authors’ information
GS is a DrPH candidate at Harvard T. H. Chan School of Public Health. RA is the director of the Global Health Systems Cluster at Harvard T. H. Chan School of Public Health.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ART
Antiretroviral therapy
- CHW
Community health worker
- DOTS
Directly observed treatment—short course
- HSS
Health systems strengthening
- ISPA
Indirectly supervised pharmacist assistant
- LMIC
Low-income and middle-income countries
- MSM
Men who have sex with men
- NCD
Non-communicable disease
- NTD
Neglected tropical disease
- PHC
Primary health care
- RMNCH
Reproductive, maternal, newborn, and child health
- TB
Tuberculosis
- VHW
Village health worker
- WHO
World Health Organization
Contributor Information
Gabriel Seidman, Email: Gabriel.seidman@gmail.com.
Rifat Atun, Email: ratun@hsph.harvard.edu.
References
- 1.Grigoli F, Kapsoli J. Waste not, want not: the efficiency of health expenditure in emerging and developing economies. IMF Working Papers. 2013;(187).
- 2.Hernandez-Peña P. Health worker remuneration in WHO Member States. Bull World Health Organ. 2013;91(11):808–815. doi: 10.2471/BLT.13.120840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chisholm D, Evans DB. Improving health system efficiency as a means of moving towards universal coverage, in World Health Report Background Paper. Geneva: World Health Organization; 2010.
- 4.World Health Organization. First Global Conference on Task Shifting. 2008 [cited 2016 March 15]; Available from: http://www.who.int/healthsystems/task_shifting/en/.
- 5.Joshi R, Alim M, Kengne AP, Jan S, Maulik PK, Peiris D, Patel AA. Task shifting for non-communicable disease management in low and middle income countries—a systematic review. PLoS One. 2014;9(8):e103754. doi: 10.1371/journal.pone.0103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kredo T, Adeniyi FB, Bateganya M, Pienaar ED. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. Cochrane Database Syst Rev. 2014;7:Cd007331. doi: 10.1002/14651858.CD007331.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penazzato M, Davies MA, Apollo T, Negussie E, Ford N. Task shifting for the delivery of pediatric antiretroviral treatment: a systematic review. J Acquir Immune Defic Syndr. 2014;65(4):414–22. doi: 10.1097/QAI.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 8.Polus S, Lewin S, Glenton C, Lerberg PM, Rehfuess E, Gülmezoglu AM. Optimizing the delivery of contraceptives in low- and middle-income countries through task shifting: a systematic review of effectiveness and safety. Reprod Health. 2015;12:27. doi: 10.1186/s12978-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-González NA, Tandjung R, Djalali S, Rosemann T. The impact of physician-nurse task shifting in primary care on the course of disease: a systematic review. Hum Resour Health. 2015;13:55. doi: 10.1186/s12960-015-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughan K. Costs and cost-effectiveness of community health workers: evidence from a literature review. Hum Resour Health. 2015;13(1):1. doi: 10.1186/s12960-015-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PRISMA. PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses. 2015 [cited 2016 September 6]; Available from: http://www.prisma-statement.org/.
- 12.W. K. Kellogg Foundation . Logic Model Development Guide. Michigan: Battle Creek; 2004. [Google Scholar]
- 13.Alam K, Khan JA, Walker DG. Impact of dropout of female volunteer community health workers: an exploration in Dhaka urban slums. BMC Health Serv Res. 2012;12:260. doi: 10.1186/1472-6963-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghi J. Economic assessment of a women's group intervention to improve birth outcomes in rural Nepal. Lancet. 2005;366(9500):1882–1884. doi: 10.1016/S0140-6736(05)67758-6. [DOI] [PubMed] [Google Scholar]
- 15.Bowser D, et al. A cost-effectiveness analysis of community health workers in Mozambique. J Pri Care Commun Health. 2015;6(4). [DOI] [PubMed]
- 16.Chin-Quee D. Building on safety, feasibility, and acceptability: the impact and cost of community health worker provision of injectable contraception. Global Health Sci Pract. 2013;1(3):316–327. doi: 10.9745/GHSP-D-13-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chola L. Cost of individual peer counselling for the promotion of exclusive breastfeeding in Uganda. Cost Eff Resour Alloc. 2011;9(1):1. doi: 10.1186/1478-7547-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onwujekwe O, Uzochukwu B, Ojukwu J, Dike N, Shu E. Feasibility of a community health worker strategy for providing near and appropriate treatment of malaria in southeast Nigeria: an analysis of activities, costs and outcomes. Acta Trop. 2007;101(2):95–105. doi: 10.1016/j.actatropica.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Prinja S, Jeet G, Verma R, Kumar D, Bahuguna P, Kaur M, Kumar R. Economic analysis of delivering primary health care services through community health workers in 3 North Indian states. PLoS One. 2014;9(3):e91781. doi: 10.1371/journal.pone.0091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prinja S, Mazumder S, Taneja S, Bahuguna P, Bhandari N, Mohan P, Hombergh H, Kumar R. Cost of delivering child health care through community level health workers: how much extra does IMNCI program cost? J Trop Pediatr. 2013;59(6):489–95. doi: 10.1093/tropej/fmt057. [DOI] [PubMed] [Google Scholar]
- 21.Sabin LL. Costs and cost-effectiveness of training traditional birth attendants to reduce neonatal mortality in the Lufwanyama Neonatal Survival study (LUNESP) PLoS One. 2012;7(4):e35560. doi: 10.1371/journal.pone.0035560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conteh L. Cost effectiveness of seasonal intermittent preventive treatment using amodiaquine & artesunate or sulphadoxine-pyrimethamine in Ghanaian children. PLoS One. 2010;5(8):e12223. doi: 10.1371/journal.pone.0012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmud N, Rodriguez J, Nesbit J. A text message-based intervention to bridge the healthcare communication gap in the rural developing world. Technol Health Care. 2010;18(2):137–44. doi: 10.3233/THC-2010-0576. [DOI] [PubMed] [Google Scholar]
- 24.Nonvignon J, Chinbuah MA, Gyapong M, Abbey M, Awini E, Gyapong JO, Aikins M. Is home management of fevers a cost-effective way of reducing under-five mortality in Africa? The case of a rural Ghanaian District. Trop Med Int Health. 2012;17(8):951–7. doi: 10.1111/j.1365-3156.2012.03018.x. [DOI] [PubMed] [Google Scholar]
- 25.Gaziano TA, Bertram M, Tollman SM, Hofman KJ. Hypertension education and adherence in South Africa: a cost-effectiveness analysis of community health workers. BMC Public Health. 2014;14:240. doi: 10.1186/1471-2458-14-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCord GC, Liu A, Singh P. Deployment of community health workers across rural sub-Saharan Africa: financial considerations and operational assumptions. Bull World Health Organ. 2013;91(4):244–53b. doi: 10.2471/BLT.12.109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland T, Bishai DM. Cost-effectiveness of misoprostol and prenatal iron supplementation as maternal mortality interventions in home births in rural India. Int J Gynecol Obstet. 2009;104(3):189–193. doi: 10.1016/j.ijgo.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Tozan Y, Klein EY, Darley S, Panicker R, Laxminarayan R, Breman JG. Pre-referral rectal artesunate is cost-effective for treating severe childhood malaria. Am J Trop Med Hyg. 2009;81(5):305. [Google Scholar]
- 29.Fiedler JL. A cost analysis of the Honduras community-based integrated child care program. Health, Nutrition and Population (HNP) Discussion Paper. Washington: The World Bank; 2003. [Google Scholar]
- 30.Johns B. Assessing the costs and effects of antiretroviral therapy task shifting from physicians to other health professionals in Ethiopia. J Acquir Immune Defic Syndr. 2014;65(4):e140–7. doi: 10.1097/QAI.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 31.Johns, B. and E. Baruwa, The effects of decentralizing anti-retroviral services in Nigeria on costs and service utilization: two case studies. Health Policy Plan. 2015;31(2):182–191. [DOI] [PubMed]
- 32.Mbonye A. Intermittent preventive treatment of malaria in pregnancy: the incremental cost-effectiveness of a new delivery system in Uganda. Trans R Soc Trop Med Hyg. 2008;102(7):685–693. doi: 10.1016/j.trstmh.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Hounton SH. A cost-effectiveness study of caesarean-section deliveries by clinical officers, general practitioners and obstetricians in Burkina Faso. Hum Resour Health. 2009;7(1):34. doi: 10.1186/1478-4491-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munyaneza F. Leveraging community health worker system to map a mountainous rural district in low resource setting: a low-cost approach to expand use of geographic information systems for public health. Int J Health Geogr. 2014;13:49. doi: 10.1186/1476-072X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datiko DG, Lindtjorn B. Cost and cost-effectiveness of smear-positive tuberculosis treatment by Health Extension Workers in Southern Ethiopia: a community randomized trial. PLoS One. 2010;5(2):e9158. doi: 10.1371/journal.pone.0009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam MA. Cost-effectiveness of community health workers in tuberculosis control in Bangladesh. Bull World Health Organ. 2002;80(6):445–50. [PMC free article] [PubMed] [Google Scholar]
- 37.Dick J. Primary health care nurses implement and evaluate a community outreach approach to health care in the South African agricultural sector. Int Nurs Rev. 2007;54(4):383–90. doi: 10.1111/j.1466-7657.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 38.Okello D. Cost and cost-effectiveness of community-based care for tuberculosis patients in rural Uganda. Int J Tuberc Lung Dis. 2003;7(9s1):S72–S79. [PubMed] [Google Scholar]
- 39.Clarke M, Dick J, Bogg L. Cost-effectiveness analysis of an alternative tuberculosis management strategy for permanent farm dwellers in South Africa amidst health service contraction. Scand J Public Health. 2006;34(1):83–91. doi: 10.1080/14034940510032220. [DOI] [PubMed] [Google Scholar]
- 40.Khan MA. Costs and cost-effectiveness of different DOT strategies for the treatment of tuberculosis in Pakistan. Directly Observed Treatment. Health Policy Plan. 2002;17(2):178–86. doi: 10.1093/heapol/17.2.178. [DOI] [PubMed] [Google Scholar]
- 41.Floyd K. Cost and cost-effectiveness of increased community and primary care facility involvement in tuberculosis care in Lilongwe District, Malawi. Int J Tuberc Lung Dis. 2003;7(9):S29–S37. [PubMed] [Google Scholar]
- 42.Prado TN. Cost-effectiveness of community health worker versus home-based guardians for directly observed treatment of tuberculosis in Vitoria, Espirito Santo State, Brazil. Cad Saude Publica. 2011;27(5):944–52. doi: 10.1590/S0102-311X2011000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinanovic E. Cost and cost-effectiveness of community-based care for tuberculosis in Cape Town, South Africa. Int J Tuberc Lung Dis. 2003;7(9s1):S56–S62. [PubMed] [Google Scholar]
- 44.Yan H. The increased effectiveness of HIV preventive intervention among men who have sex with men and of follow-up care for people living with HIV after ‘task-shifting’ to community-based organizations: a 'cash on service delivery' model in China. PLoS One. 2014;9(7):e103146. doi: 10.1371/journal.pone.0103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fatti G, Monteith L, Shaikh N, Kapp E, Foster N, Grimwood A., A Comparison of Two Task-Shifting Models of Pharmaceutical Care in Antiretroviral Treatment Programs in South Africa. J Acquir Immune Defic Syndr. 2015;71(4). [DOI] [PubMed]
- 46.Babigumira JB. Cost effectiveness of a pharmacy-only refill program in a large urban HIV/AIDS clinic in Uganda. PLoS One. 2011;6(3):e18193. doi: 10.1371/journal.pone.0018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster N, McIntyre D. Economic evaluation of task-shifting approaches to the dispensing of anti-retroviral therapy. Hum Resour Health. 2012;10:32. doi: 10.1186/1478-4491-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bemelmans M. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health. 2014;19(8):968–977. doi: 10.1111/tmi.12332. [DOI] [PubMed] [Google Scholar]
- 49.Chanda P. Relative costs and effectiveness of treating uncomplicated malaria in two rural districts in Zambia: Implications for nationwide scale-up of home-based management. Malar J. 2011;10:159. doi: 10.1186/1475-2875-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamainza B, Moonga H, Sikaala CH, Kamuliwo M, Bennett A, Eisele TP, Miller J, Seyoum A, Killeen GF. Monitoring, characterization and control of chronic, symptomatic malaria infections in rural Zambia through monthly household visits by paid community health workers. Malar J. 2014;13:128. doi: 10.1186/1475-2875-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patouillard E. Coverage, adherence and costs of intermittent preventive treatment of malaria in children employing different delivery strategies in Jasikan. Ghana PloS one. 2011;6(11):e24871. doi: 10.1371/journal.pone.0024871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruebush TK., 2nd Community participation in malaria surveillance and treatment. III. An evaluation of modifications in the Volunteer Collaborator Network of Guatemala. Am J Trop Med Hyg. 1994;50(1):85–98. doi: 10.4269/ajtmh.1994.50.85. [DOI] [PubMed] [Google Scholar]
- 53.Sikaala CH. A cost-effective, community-based, mosquito-trapping scheme that captures spatial and temporal heterogeneities of malaria transmission in rural Zambia. Malar J. 2014;13:225. doi: 10.1186/1475-2875-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jafar TH. Cost-effectiveness of community-based strategies for blood pressure control in a low-income developing country: findings from a cluster-randomized, factorial-controlled trial. Circulation. 2011;124(15):1615–25. doi: 10.1161/CIRCULATIONAHA.111.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puett C. Cost-effectiveness of the community-based management of severe acute malnutrition by community health workers in southern Bangladesh. Health Policy Plan. 2013;28(4):386–99. doi: 10.1093/heapol/czs070. [DOI] [PubMed] [Google Scholar]
- 56.Laveissiere C. Detecting sleeping sickness: comparative efficacy of mobile teams and community health workers. Bull World Health Organ. 1998;76(6):559–64. [PMC free article] [PubMed] [Google Scholar]
- 57.Buttorff C. Economic evaluation of a task-shifting intervention for common mental disorders in India. Bull World Health Organ. 2012;90(11):813–21. doi: 10.2471/BLT.12.104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kruk ME. Economic evaluation of surgically trained assistant medical officers in performing major obstetric surgery in Mozambique. Bjog. 2007;114(10):1253–60. doi: 10.1111/j.1471-0528.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 59.Sadruddin S. Household costs for treatment of severe pneumonia in Pakistan. Am J Trop Med Hyg. 2012;87(5 Suppl):137–43. doi: 10.4269/ajtmh.2012.12-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuit R. Result of a first step toward community-based surveillance of transmission of Chagas’ disease with appropriate technology in rural areas. Am J Trop Med Hyg. 1992;46(4):444–50. doi: 10.4269/ajtmh.1992.46.444. [DOI] [PubMed] [Google Scholar]
- 61.Cline BL, Hewlett BS. Community-based approach to schistosomiasis control. Acta Trop. 1996;61(2):107–19. doi: 10.1016/0001-706X(95)00118-X. [DOI] [PubMed] [Google Scholar]
- 62.Aung T. Incidence of pediatric diarrhea and public-private preferences for treatment in rural Myanmar: a randomized cluster survey. J Trop Pediatr. 2013;59(1):10–16. doi: 10.1093/tropej/fms033. [DOI] [PubMed] [Google Scholar]
- 63.Fiedler JL, Villalobos CA, De Mattos AC. An activity-based cost analysis of the Honduras community-based, integrated child care (AIN-C) programme. Health Policy Plan. 2008;23(6):408–427. doi: 10.1093/heapol/czn018. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Tuberculosis: Fact Sheet No. 104. 2015 [cited 2016 March 14]; Available from: http://www.who.int/mediacentre/factsheets/fs104/en/.
- 65.Kim, J.Y. The burden of tuberculosis: Economic burden (2). 2016 [cited 2016 September 15]; Available from: http://who.int/trade/distance_learning/gpgh/gpgh3/en/index7.html.
- 66.UNAIDS. Fact Sheet 2016. 2016 [cited 2016 September 16]; Available from: http://www.unaids.org/en/resources/fact-sheet.
- 67.UNAIDS. Fast-Track Update on Investments Needed in the AIDS Response. 2016 [cited 2016 September 15]; Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Reference_FastTrack_Update_on_investments_en.pdf.
- 68.World Health Organization, Classifying health workers: Mapping occupations to the international standard classification. Geneva: World Health Organization.
- 69.Jaskiewicz W, Tulenko K. Increasing community health worker productivity and effectiveness: a review of the influence of the work environment. Hum Resour Health. 2012;10(1):1. doi: 10.1186/1478-4491-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glenton C, et al. Barriers and facilitators to the implementation of lay health worker programmes to improve access to maternal and child health: qualitative evidence synthesis. Cochrane Database Syst Rev. 2013;10:Cd010414. [DOI] [PMC free article] [PubMed]
- 71.Pallas SW. Community health workers in low- and middle-income countries: what do we know about scaling up and sustainability? Am J Public Health. 2013;103(7):e74–e82. doi: 10.2105/AJPH.2012.301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bastawrous A, Giardini ME, Bolster NM, Peto T, Shah N, Livingstone IA, Weiss HA, Hu S, Rono H, Kuper H, Burton M. Clinical Validation of a Smartphone-Based Adapter for Optic Disc Imaging in Kenya. JAMA Ophthalmol. 2016;134(2). [DOI] [PMC free article] [PubMed]
- 73.Gupta B, Huckman RS, Khanna T. Task shifting in surgery: lessons from an Indian Heart Hospital. Healthc (Amst) 2015;3(4):245–50. doi: 10.1016/j.hjdsi.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Boullé C, Kouanfack C, Laborde-Balen G, Carrieri MP, Dontsop M, Boyer S, Aghokeng AF, Spire B, Koulla-Shiro S, Delaporte E, Laurent C. Task shifting HIV care in rural district hospitals in Cameroon: evidence of comparable antiretroviral treatment-related outcomes between nurses and physicians in the Stratall ANRS/ESTHER trial. J Acquir Immune Defic Syndr. 2013;62(5):569–76. doi: 10.1097/QAI.0b013e318285f7b6. [DOI] [PubMed] [Google Scholar]
- 75.Nash D, Azeez S, Vlahov D, Schori M. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83(2):231–43. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mullan F, Frehywot S. Non-physician clinicians in 47 sub-Saharan African countries. Lancet. 2007;370(9605):2158–63. doi: 10.1016/S0140-6736(07)60785-5. [DOI] [PubMed] [Google Scholar]
- 77.Chu K. Surgical task shifting in sub-Saharan Africa. PLoS Med. 2009;6(5):e1000078. doi: 10.1371/journal.pmed.1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Key information from original dataset included in Table 1 is in the manuscript. The original dataset is available from the corresponding author upon request.


