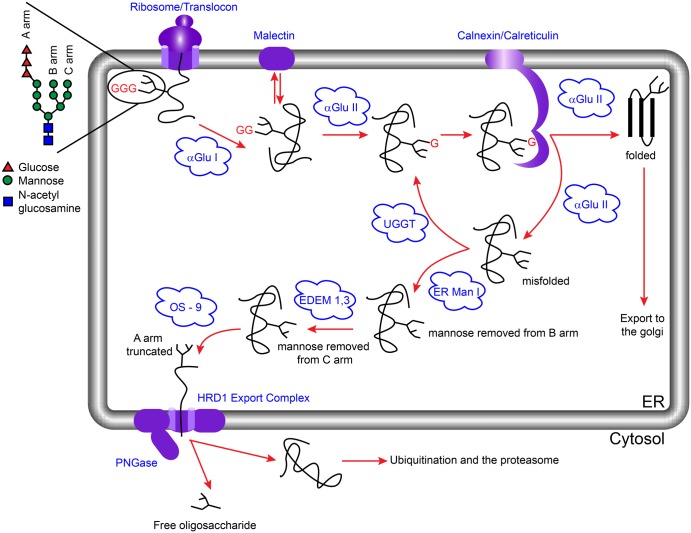
Figure 2. The calnexin cycle and ERAD.
The precursor glycan Glc3Man9GlcNAc2 (represented here for simplicity with the glucose residues as red triangles and the remaining portion of the glycan shown as black lines) is added to a peptide co-translationally. Cleavage of the terminal glucose residue by α-glu I leads to a form that can either bind to malectin or be further trimmed by α-glu II to become a substrate for calnexin/calreticulin. On release from calnexin/calreticulin, α-glu II can remove the remaining glucose residue. At this point properly folded proteins are exported to the Golgi for further processing, whilst misfolded proteins are either reglucosylated by UGGT for a ‘second chance’ at folding or directed to the ERAD pathway by ER mannosidase I (ER Man I), which removes a mannose residue from the B-arm of the glycan [42,79]. ER degradation-enhancing α-mannosidase-like proteins 1–3 (EDEM1–3) then act on the C-arm of the glycan followed by OS-9/XTP3-B-mediated delivery of the substrate to the Hrd1 ubiquitination complex through the interaction with a membrane-spanning adaptor protein, SEL1L [80–87]. PNGase separates the glycan from the protein and both segments are degraded [44,88].