Fig. 6.
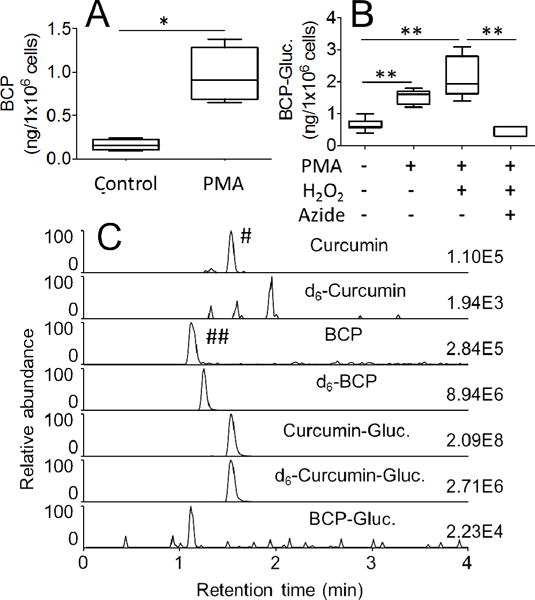
Oxidative transformation of curcumin and curcumin-glucuronide by leukocytes isolated from human blood. (A) Leukocytes were pretreated with phorbol ester (PMA) or vehicle (control) for 30 min, and oxidation of curcumin to bicyclopentadione was quantified by LC-MS. (B) Leukocytes were pretreated with vehicle or phorbol ester (PMA) in the presence or absence of H2O2 (40 μM) and sodium azide (100 μM), and oxidation of curcumin-glucuronide to bicyclopentadione-glucuronide was quantified by LC-MS. (C) SRM-ion traces for the analysis of curcumin, d6-curcumin, bicyclopentadione (BCP), d6-bicyclopentadione (d6-BCP), curcumin-glucuronide (Curcumin-Gluc.), d6-curcumin-glucuronide (d6-Curcumin-Gluc.) and bicyclopentadione-glucuronide (BCP-Gluc.) are shown. Formation of the two peaks marked with # and ## is explained in the Results section. * and ** represent statistically significant differences between incubations with p<0.05 and p<0.01, respectively (n = 3). Whiskers in boxplot represent minimum and maximum values.
