Abstract
Background
There are limited data regarding the prevalence, pathophysiology and management implications of pulmonary hypertension in patients with obstructive hypertrophic cardiomyopathy (HCM) and advanced heart failure.
Methods and Results
To assess the clinical significance of measured cardiopulmonary hemodynamics in HCM patients with heart failure, we retrospectively assessed right heart catheterization data in 162 consecutive patients with outflow tract gradients (median [interquartile range]) (90 [70–110] mmHg), 59±11 years old, and 49% male, predominately NYHA Class III/IV status. Pulmonary hypertension (mean pulmonary artery pressure [mPAP] ≥25 mmHg) was present in 82 patients (51%), including 29 (18%) regarded as moderate-severe (mPAP ≥35 mmHg) and 28 (34%) that also had increased pulmonary vascular resistance (PVR) >3.0 WU. The pulmonary artery wedge pressure (PAWP) was ≤15 mmHg in 54%, indicating that left atrial hypertension was absent in a majority of patients. Notably, 9 patients (11%) met hemodynamic criteria for pre-capillary pulmonary hypertension (mPAP ≥25 mmHg, PVR >3.0 WU, PAWP ≤15 mmHg). Over a median follow-up of 327 [90–743] days after surgical myectomy (or alcohol septal ablation), 92% and 95% of patients with or without preoperative pulmonary hypertension, respectively, were asymptomatic or mildly symptomatic. One postoperative death occurred in a 59-year old woman with acute respiratory failure and mPAP=65 mmHg.
Conclusions
Pulmonary hypertension was common in obstructive HCM patients with advanced heart failure. While possibly a contributor to pre-operative heart failure, pulmonary hypertension did not significantly influence clinical and surgical outcome. Notably, a novel patient subgroup was identified with resting invasive hemodynamics consistent with pulmonary vascular disease.
Keywords: hypertrophic cardiomyopathy, pulmonary heart disease, myectomy
Subject Terms: Cardiomyopathy, Pathophysiology, Pulmonary Hypertension
Heart failure in Hypertrophic Cardiomyopathy (HCM) is most commonly due to mechanical impedance to left ventricular (LV) outflow, present in up to 70% of patients at rest or with physiologic provocation, and produced by mitral valve systolic anterior motion with associated mitral regurgitation, resulting in elevated LV pressures and left atrial hypertension.1,2 However, the prevalence, clinical significance and management implications of pulmonary hypertension and the associated risk for adverse outcomes in HCM patients are incompletely understood. Prior reports characterizing pulmonary artery pressure dynamics in obstructive HCM patients have relied largely on estimates derived from Doppler echocardiography studies, and attributed adverse consequences (i.e., all-cause mortality) to pulmonary hypertension.3,4 Therefore, in the present analysis we have re-visited clinical issues surrounding pulmonary hypertension in a unique cohort of HCM patients using hemodynamic data obtained at cardiac catheterization.
Methods
Study population
We evaluated 187 consecutive adult patients diagnosed with obstructive HCM who underwent right heart catheterization and subsequent septal reduction with surgical myectomy or percutaneous alcohol septal ablation at Tufts Medical Center, 2009–2015. Diagnosis of HCM was based on echocardiographic and/or cardiovascular magnetic resonance imaging evidence of LV hypertrophy (wall thickness ≥15 mm) involving ≥1 segment of the chamber in the absence of a cardiac or systemic disease capable of producing the extent of hypertrophy evident. Twenty-five patients were excluded from this analysis because of moderate or severe mitral or aortic disease unrelated to HCM, and the final study group comprised 162 patients. This study has been reviewed and approved by Institutional Review Boards of Tufts Medical Center (IRB# 12019) for retrospective collection of data for this project; the requirement of informed consent was waived for all patients.
Echocardiography
Transthoracic echocardiography was performed using commercially available instruments. Measurements of LV wall thickness and cavity dimensions, ejection fraction, and LV outflow tract gradient (estimated with continuous wave Doppler) were obtained as previously reported.5 Mitral regurgitation was graded mild, moderate, and severe using semi-quantitative criteria. Left atrial volume was calculated with the biplane area-length method and indexed to body surface area. Patients with LV outflow tract gradient of zero or <50 mmHg under resting conditions underwent stress echocardiography with upright exercise on a symptom-limited Bruce protocol; subaortic gradient was assessed at baseline and immediately after exercise in the supine position. Measurements of right ventricular diameter and systolic function including fractional area change and peak systolic tricuspid annular velocity (S’) were measured according to standard published guidelines.6
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (CMR) studies were performed in 118 of the 162 HCM study patients (73%) using a 1.5 T clinical scanner. Cine sequences were performed in standard views with full LV coverage. Late gadolinium enhancement (LGE) images were acquired 10–15 minutes after intravenous administration of 0.2 mmol/kg gadolinium-DTPA using breath-held segmented inversion-recovery sequence. LGE quantification was performed by manually adjusting grayscale threshold to visually define LGE, which were summed and expressed as proportion of total LV myocardium.7
Cardiac catheterization
Each patient underwent right heart catheterization in the supine position at rest, either as part of the pre-myectomy clinical evaluation or at the time of alcohol septal ablation. Most patients continued to take medications that had been previously administered for the purpose of controlling symptoms of heart failure (e.g., beta-adrenergic receptor blockers or verapamil).
Right atrial, mean pulmonary artery pressure (mPAP), and pulmonary artery wedge pressure (PAWP) were measured at end-expiration and averaged over ≥3 beats. Cardiac output (CO), measured in 157 patients, was determined by the thermodilution (N=150) or assumed Fick (n=7) methods, and indexed to body surface area. Pulmonary vascular resistance (PVR) was calculated as: (mPAP-PAWP)/CO, transpulmonary gradient (TPG) was calculated as: mPAP-PAWP, and diastolic pressure gradient (DPG) was calculated as (diastolic PAP – PAWP).
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) for normally distributed variables or as median [interquartile range] for non-normally distributed variables. Comparisons between normally distributed groups were performed by Student’s t-test. The Mann-Whitney or Kruskal-Wallis tests were used for comparisons between non-normally distributed variables. Exact Chi-squared test was used for categorical variables. All tests were two-tailed and statistical significance was defined by P< 0.05. All analyses were performed using R Version 3.2.5.8
Results
Study population
The 162 study patients with obstructive HCM and septal reduction therapy (surgical myectomy in 116 and alcohol septal ablation in 46) were 35 to 82 years of age (mean 59 ± 11 years); 79 (49%) were male (Table 1). Maximum LV wall thickness was 19 ± 4 mm, and LV outflow obstruction (≥30 mmHg) was present at rest (n=125) or following physiologic provocation (n=37) in all patients, with peak systolic gradients of 50 to 160 mmHg (average 90 mmHg).
Table 1.
Clinical, Echocardiographic and Hemodynamic Characteristics in 162 Patients with Obstructive HCM.
| General Characteristics | |
| Age (years) | 59.2 (10.5) |
| Male gender, n (%) | 79 (49) |
| BMI (kg/m2) | 30.9 ± 6.9 |
| Atrial fibrillation, n (%) | 36 (22) |
| Medications at RHC | |
| Beta-blockers, n (%) | 135 (83) |
| Calcium-channel blockers, n (%) | 49 (30) |
| Disopyramide, n (%) | 20 (12) |
| Diuretics, n (%) | 39 (24) |
| Echocardiographic variables | |
| Peak LVOT gradient (mmHg) | 90 [70–110] |
| LV EF (%) | 65 [61–70] |
| Maximum LV wall thickness (mm) | 19.3 ± 3.9 |
| LV end-diastolic volume index (ml/m2) | 51 [43–59] |
| MR: moderate or severe, n (%) | 30 (19) |
| Left atrial volume index (ml/m2) | 42 [36–52] |
| Medial E/e’>15, n (%) | 108 (72) |
| RV diameter (mm) | 37 [33–42] |
| RV diameter > 42 mm, n (%) | 36 (22) |
| RV fractional area change (%) | 47 [43–51] |
| Peak tricuspid annulus S’ velocity* (cm/s) | 12.3 [11.0–14.0] |
| Hemodynamic variables | |
| PASP (mmHg) | 41 [31–46] |
| Mean PAP (mmHg) | 25 [20–31] |
| mPAP ≥25 mmHg, n (%) | 82 (51) |
| mPAP 25–34 mmHg, n (%) | 53 (33) |
| mPAP 35–44 mmHg, n (%) | 17 (11) |
| mPAP ≥45 mmHg, n (%) | 12 (7) |
| Right atrial pressure (mmHg) | 7 [5–10] |
| TPG (mmHg) | 10 [7–13] |
| TPG>12 mmHg, n (%) | 45 (28) |
| PAWP (mmHg) | 15 [12–20] |
| PAWP >15 mmHg, n (%) | 74 (46) |
| PVR (WU) | 1.89 [1.31–2.57] |
| PVR >3.0 WU, n (%) | 34 (21) |
| CI (L/min/m2) | 2.51 [2.17–2.91] |
| DPG ≥7 mmHg (%) | 11 (7%) |
For continuous data, results are reported as mean ± SD. For data not distributed normally, results are reported as median [interquartile range]. *Data were available for 92 of 162 patients (57%).
Abbreviations: BMI, body mass index; CI, cardiac index; LV, left ventricular; EF, ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NYHA, New York Heart Association; PAP pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PAWP pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization; TPG, transpulmonary gradient; WU, Wood Units; RV, right ventricle; DPG, diastolic pressure gradient.
Patients were severely symptomatic, consistent with New York Heart Association (NYHA) functional class III/IV, and refractory to maximum medical management prior to septal reduction. Moderate to severe mitral regurgitation was present in 30 patients (19%), associated with increased left atrial volume (≥34 ml/m2) in 28 patients; LV ejection fraction ranged from 48% to 80% (mean 65%). Thirty-six patients (22%) had ≥1 episode of symptomatic atrial fibrillation. Obstructive atherosclerotic coronary artery disease was present in 22 patients (14%). The average RV diameter (37 [33–42] mm), RV fractional area change (47 [43–51 % change), and peak tricuspid annulus S’ velocity (12.3 [11.0–14.0 cm/s] were within the range of normal.
Group hemodynamic profile
Among the 162 study patients, mPAP ranged from 11 to 65 mmHg (average 27 ± 10 mmHg); 82 patients (51%) were judged to have pulmonary hypertension with mPAP ≥25 mmHg. Of these: 36 were 25–30 mmHg, 19 were 31–35 mmHg, 9 were 36–40 mmHg and 18 were >40 mmHg (Figure 1). Of the 82 patients with increased pulmonary artery pressure, 28 (34%) also had increased PVR (>3.0 WU). The average pulmonary artery systolic pressure for the entire cohort was 41 [31–46] mmHg.
Figure 1.
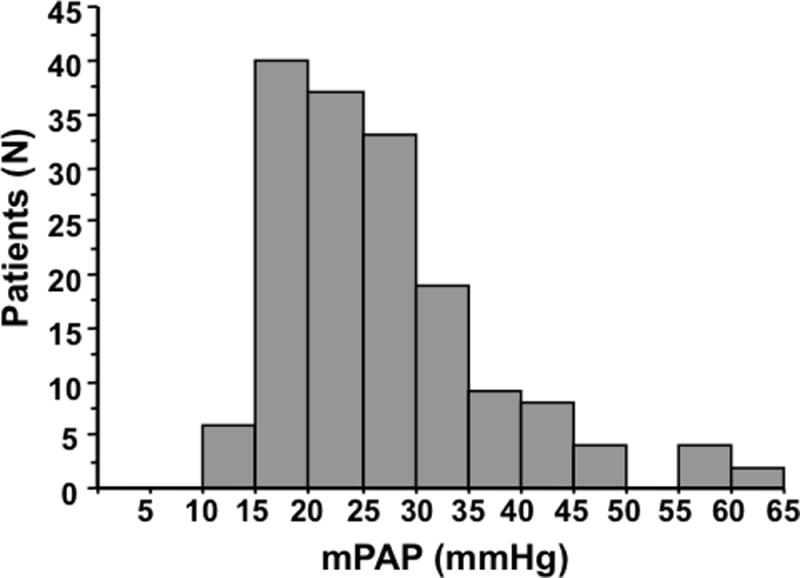
Distribution of mean pulmonary artery pressure (mPAP) in 162 HCM patients undergoing cardiac catheterization.
The mPAP did not correlate with peak LV outflow tract gradient (r=0.09, P=0.32) (Figure 2). Correlation between systolic pulmonary artery pressure measured at catheterization mmHg with that estimated by Doppler echocardiography (28 [23–32] mmHg) in 131 patients was r=0.72, P<0.001, with Doppler estimate being 9 [4–16] mmHg lower than the invasive measure. Employing suggested alternative criteria for pulmonary hypertension (mPAP ≥20 mmHg),9 132 patients (82%) could be regarded as having increased pulmonary artery pressure.
Figure 2.
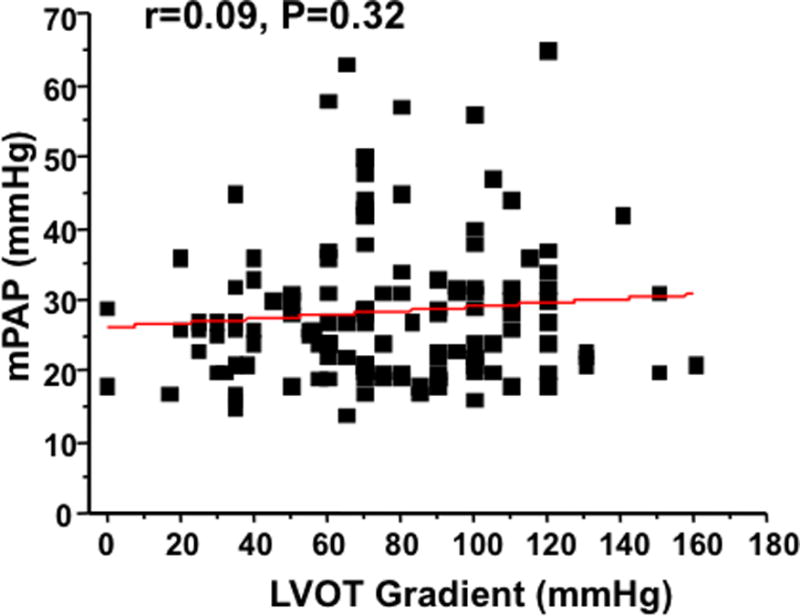
Mean pulmonary artery pressure (mPAP) does not correlate with resting left ventricular outflow tract (LVOT) gradient in patients with obstructive HCM.
The PAWP at rest ranged from 6 to 41 mmHg (average 16 ± 7 mmHg), and was >15 mmHg in 74 patients (46%). Cardiac output was measured in 157 subjects and ranged from 2.2 to 12.3 L/min (cardiac index 1.0 to 5.4 L/min/m2), normal (>5.0 L/min) in 73 patients, and reduced (<3.0 L/min) in 5 patients.
Relation of mPAP to clinical variables
Compared to patients with normal mPAP (<25 mmHg), the 82 patients with pulmonary hypertension (mPAP ≥25 mmHg) were older (61.4 vs. 56.9 years, p=0.006), with higher body mass index (33.2 to 28.6 kg/m2, p<0.001), maximum LV wall thickness (20.0 to 18.6 mm, p=0.024) and left atrial volume index (45 to 41 ml/m2) (Table 2). Clinically meaningful differences between the groups were not identified for RV diameter (36 [32–42] vs. 37 [34–42] mm, P=0.29) or RV fractional area change (48 [45–51] vs. 46 [42–50] % change, P=0.023). The prevalence of LGE did not differ between the HCM patients with pulmonary hypertension compared to those without pulmonary hypertension (51% vs. 55 %; p=0.51). In addition, among patients with LGE, there was no significant relationship between extent of LGE and mean pulmonary artery pressure (r=0.006; P=0.95). The mPAP was higher in alcohol septal ablation patients compared with myectomy patients (30 [23–41] vs. 24 [20–29] mmHg, P<0.02).
Table 2.
Clinical and Hemodynamic Characteristics with Respect to Mean Pulmonary Artery Pressure (mPAP)
| mPAP <25 mmHg (N=80) |
mPAP≥25 mmHg (N=82) |
P-value | |
|---|---|---|---|
| General Characteristics | |||
| Age (years) | 56.9 (9.6) | 61.4 (10.8) | 0.006 |
| Male gender, n (%) | 42 (52.5) | 37 (45.1) | 0.43 |
| BMI (kg/m2) | 28.6 ± 5.3 | 33.2 ± 7.5 | <0.001 |
| NYHA class 3 or 4 at RHC, n (%) | 55 (68.8) | 64 (78.1) | 0.21 |
| Atrial fibrillation, n (%) | 17 (21.3) | 19 (23.2) | 0.92 |
| Echocardiography | |||
| Peak LVOT gradient (mmHg) | 90 [75–105] | 88 [70–110] | 0.66 |
| LV EF (%) | 66 [61–71] | 64 [60–69] | 0.14 |
| Maximum wall thickness (mm) | 18.6 ± 3.8 | 20.0 ± 3.9 | 0.024 |
| Moderate or severe MR, n (%) | 12 (15) | 18 (22) | 0.31 |
| Left Atrial Volume Index (ml/m2) | 41 [35 – 48] | 45 [38 – 54] | 0.017 |
| Left Atrial Volume Index ≥34ml/m2, n (%) | 64 (80) | 75 (92) | 0.062 |
| LV diastolic E/A ratio ≥2, n (%) | 1 (1.3) | 9 (11.4) | 0.023 |
| LV diastolic medial E/e’>15, n (%) | 46 (60.5) | 62 (83.8) | 0.002 |
| RV diameter (mm) | 36 [32–42] | 37 [34–42] | 0.29 |
| RV diameter > 42 mm, n (%) | 16 (20) | 20 (24) | 0.57 |
| RV fractional area change (%) | 48 [45–51] | 46 [42–50] | 0.023 |
| Cardiopulmonary Hemodynamics | |||
| PASP (mmHg) | 31 [28–34] | 46 [41–56] | <0.001 |
| mPAP (mmHg) | 20 [18–22] | 31 [28–38] | <0.001 |
| Right atrial pressure (mmHg) | 6 [3 – 8] | 9 [7–13] | <0.001 |
| TPG (mmHg) | 8 [7–10] | 13 [9–16] | <0.001 |
| TPG>12 mmHg, n (%) | 4 (5) | 41 (50) | <0.001 |
| PAWP (mmHg) | 12 [10–14] | 20 [16–24] | <0.001 |
| PAWP>15 mmHg, n (%) | 8 (10.0) | 66 (80.5) | <0.001 |
| PVR (WU) | 1.59 [1.23–2.13] | 2.45 [1.66–3.26] | <0.001 |
| CI (L/min/m2) | 2.52 [2.21–2.96] | 2.51 [2.16–2.85] | 0.55 |
| DPG ≥7 mmHg (%) | 0 (0) | 12 (15) | <0.001 |
For continuous data, results are reported as mean ± SD. For data not distributed normally, results are reported as median [interquartile range].
Abbreviations: BMI, body mass index; CI, cardiac index; LV, left ventricular; EF, ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NYAH, New York Heart Association; PAP pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PAWP pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization; TPG, transpulmonary gradient; WU, Wood Units; RV, right ventricle; DPG, diastolic pressure gradient.
Co-morbidities that have been associated with pulmonary hypertension, including chronic obstructive pulmonary disease, obstructive sleep apnea, history of pulmonary embolism, connective tissue and interstitial lung diseases were present in 32 patients (20%), and were more common in those with mPAP ≥25 mmHg than in patients with mPAP <25 mmHg (27% vs. 13%, p=0.03).
Evidence for pre-capillary pulmonary hypertension or pulmonary arterial hypertension (PAH)
A subgroup of 9 (11%) patients differed from other study patients by virtue of normal PAWP (≤15 mmHg) in the presence of PVR >3.0 W.U. The majority did not have concomitant clinical comorbidites or significant mitral regurgitation that could be responsible for increased pulmonary artery pressures. These 9 patients were older (68 vs. 61 years, p=0.042) than others with pulmonary hypertension, but did not significantly differ from other pulmonary hypertension patients with respect to gender, LV outflow gradient, ejection fraction, maximum wall thickness, left atrial volume and mPAP. However, a higher percentage of patients in this subgroup had enlargement of the RV cavity, defined by a diameter >42 mm (20% vs. 56%, P=0.03), and RV systolic function was decreased (43 [40–46] vs. 48 [45–51 vs. % fractional area change, P=0.009) compared to other patients with HCM (Table 3).
Table 3.
Clinical Characteristics in 9 Patients with Hemodynamics Consistent with Pre-capillary pulmonary hypertension.
| Pre-capillary PH or PAH (N=9) |
|
|---|---|
| Clinical Characteristics | |
| Age (yr) | 68.3 (10.3) |
| Males, n (%) | 4 (44) |
| BMI (kg/m2) | 29.3 ± 3 |
| Active smokers, n (%) | 1 (11) |
| NYHA class 3 or 4, n (%) | 8 (89) |
| Obstructive CAD, n (%) | 3 (33) |
| Hypertension, n (%) | 2 (22) |
| Any PH-related co-morbidity, n (%) | 1 (11) |
| Medications at RHC | |
| Beta-blockers, n (%) | 8 (89) |
| Calcium-channel blockers, n (%) | 3 (33) |
| Disopyramide, n (%) | 0 (0) |
| Diuretics, n (%) | 3 (33) |
| Echocardiography | |
| LVOT Gradient at rest (mmHg) | 70 [60–80] |
| Peak LVOT Gradient (mmHg) | 80 [70–110] |
| LV EF (%) | 63 [61–72] |
| Maximum LV thickness (mm) | 20 ± 4 |
| MR moderate or severe, n (%) | 4 (44) |
| LA Volume Index (ml/m2) | 46 [41–59] |
| RV diameter (mm) | 43 [33–43] |
| RV diameter > 42 mm (%) | 56 |
| RV fractional area change (%) | 43 [40–46] |
| Cardiopulmonary Hemodynamics | |
| mPAP (mmHg) | 28 [27–34] |
| TPG (mmHg) | 18 [14–19] |
| PAWP (mmHg) | 13 [12–14] |
| PVR (WU) | 3.44 [3.27–4.75] |
| CI (L/m2) | 2.07 [1.92–2.37] |
| DPG ≥7 mmHg (%) | 4 (44) |
| Invasive therapy | |
| Surgical Myectomy | N=5 |
| Alcohol Septal Ablation | N=4 |
| Major Complications | N=0 |
| Post-operative NYHA I | N=7 |
| Post-operative NYHA II | N=2 |
For normally distributed data, results are reported as mean ± SD. For data not distributed normally, results are reported as median [interquartile range].
Abbreviations: BMI, body mass index; CI, cardiac index; LV, left ventricular; EF, ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NYAH, New York Heart Association; PAP pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PAWP pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; WU, Wood Units; PH, pulmonary hypertension; DPG, diastolic pressure gradient.
Septal reduction therapy and outcome
Preoperative or preprocedural outflow gradients at rest (n=125) or with physiologic provocation (n=37) ranged from 50 to 160 mmHg (median 90). Postoperatively, at rest, each had marked reduction or obliteration of mitral valve systolic anterior motion with zero gradient or a gradient estimated <30 mmHg with Doppler echocardiography. Over a median follow-up of 327 days after myectomy or ablation, the vast majority of patients with preoperative pulmonary hypertension were asymptomatic or mildly symptomatic (n=71/77; 92%), similar to patients without pulmonary hypertension (n=77/80; 96%).
In addition, myectomy patients with severe pulmonary hypertension (mPAP ≥36 mmHg) preoperatively (N=12) did not differ significantly compared to patients with mild to moderate pulmonary hypertension (mPAP <36 mmHg) preoperatively (N=104) for relevant postoperative complications, including stroke, myocardial infarction, major bleeding, respiratory failure, acute renal failure or death; prolonged intravenous inotrope use post-operatively ≥3 days; or prolonged inpatient hospitalization (≥10 days) (2/12 [16.7%] vs. 14/104 [13.5%], P=0.67). The one post-operative death in the group with pulmonary hypertension occurred in a 59-year old woman who died from acute respiratory failure with severe pulmonary hypertension (mPAP=65 mmHg). A second post-operative death occurred in a 50-year old man due to mesenteric ischemia (mPAP=22 mmHg).
Discussion
With the decrease in sudden death events in HCM due to penetration of the implantable cardioverter defibrillator into the management of this patient population, heart failure and its consequences has assumed an enlarging profile.10,11 The mechanisms of advanced heart failure in HCM are diverse, including a minority of patients who evolve to the end-stage with or without systolic dysfunction.10,12 However, in the vast majority of patients with progressive drug-refractory disability, symptoms are related to dynamic LV outflow tract obstruction resulting in high LV pressure and wall stress.
The role of pulmonary hypertension in this HCM-related heart failure scenario is unresolved, with some investigators attributing deleterious clinical consequences (including an increase in all-cause mortality) to elevations in pulmonary pressures estimated non-invasively with Doppler echocardiography.4 On the other hand, we have the unique opportunity to report here the role of pulmonary hypertension in advanced heart failure with cardiopulmonary hemodynamics measured directly at cardiac catheterization.13 The study group comprises a consecutive cohort of patients undergoing surgical septal myectomy (or alcohol septal ablation) to relieve outflow tract obstruction and mitigate heart failure symptoms, at a single HCM tertiary referral institution.
We encountered a high frequency of elevated pulmonary artery pressures in about one-half of our study patients using the conventional criterion of mPAP ≥25 mmHg, including 15% of these patients who had particularly marked pulmonary artery pressure >45 mmHg. Furthermore, recent data in large non-HCM populations suggest that mPAP ≥20 mmHg is independently associated with adverse clinical outcome; using this cut-off value, more than 80% of our HCM patients would be considered to have pulmonary hypertension.9
Given the high frequency of pulmonary hypertension in this cohort (including moderate to severe levels), we cannot exclude the novel possibility that increased pulmonary pressures played a role in producing advanced heart failure symptoms, in association with LV outflow obstruction. Notably, we found little evidence that abnormal cardiopulmonary hemodynamics, including increased pulmonary artery pressure, were associated with deleterious clinical consequences in patients undergoing septal myectomy (or alcohol septal ablation) procedures, perioperatively, or over follow-up. For example, >95% of our patients reported improvement in symptoms from NYHA functional class III/IV to absent or only mild symptoms. However, the subgroup of patients with severe preoperative pulmonary hypertension were no more likely to experience a number of clinically relevant adverse postoperative measures such as major surgical complications, prolonged use of intravenous inotropes or extended duration of hospitalization compared to patients with mild or moderate pulmonary hypertension. One exception was a 59-year old woman who died postoperatively due to respiratory arrest with severe preoperative pulmonary hypertension (mPAP, 65 mmHg). Whether pulmonary hypertension was directly responsible for demise of this patient remains unresolved.
Overall our observations also suggest that it is unnecessary to consider specific therapeutic pharmacologic interventions to mitigate pulmonary hypertension preoperatively in HCM patients undergoing surgical or percutaneous interventions. Therefore, although pulmonary hypertension is an established risk factor for adverse outcome in patients undergoing most forms of cardiac surgery,14 our data would suggest that septal myectomy may be an exception in this regard. Nevertheless, the data reported here do not exclude the possibility that pulmonary hypertension could otherwise have a significant role in the presentation, clinical consequences and natural history of HCM, thereby underscoring the potential value of continued investigations.
Heart failure in HCM differs fundamentally in expression from that of ischemic heart disease or other non-ischemic cardiomyopathies by virtue of its characteristic association with preserved ejection fraction and normal cardiac output. An uncommon exception to this construct in HCM is the end-stage phase with impaired systolic function due to extensive myocardial scarring.1,2 The mechanism by which increased pulmonary arterial pressures develop and potentially contribute to heart failure symptoms in HCM patients with LV outflow tract obstruction remains incompletely resolved. However, it is likely that on some of our patients, the outflow tract gradient and increased LV cavitary pressure, and possibly mitral regurgitation may cause left atrial pressure to increase (inferred from the increased PAWP), and as a consequence raised pulmonary artery pressures, which in turn contributed to advanced heart failure symptoms (Figure 3).
Figure 3.
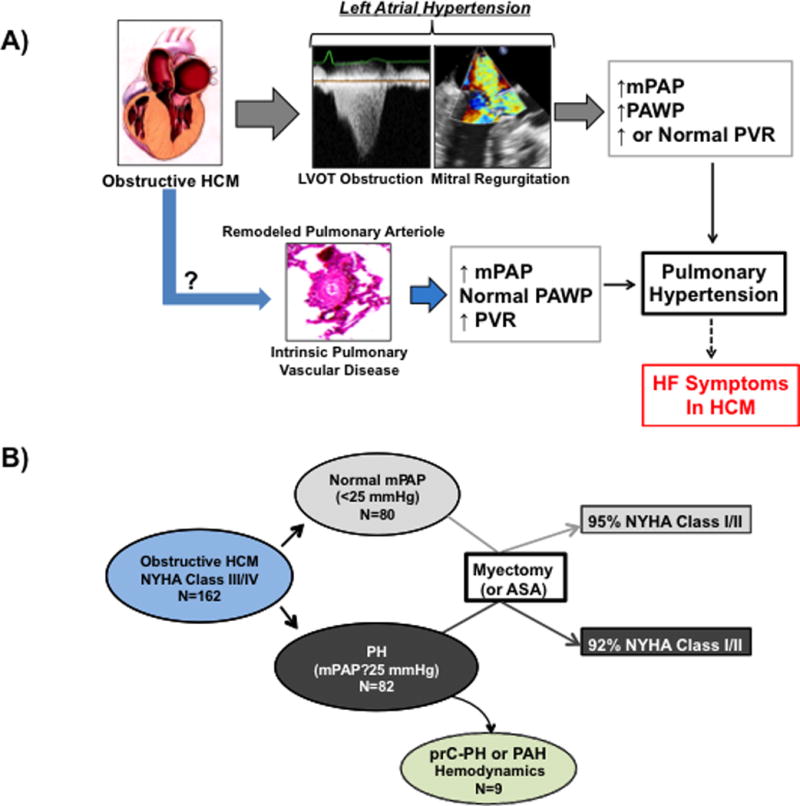
Pulmonary hypertension and the pathophysiology of heart failure symptoms in obstructive HCM. (A) Our findings suggest that in patients with obstructive HCM referred for septal reduction therapy, pulmonary hypertension is common and may be one pathophysiology underlying heart failure symptoms in this patient population. Analysis of invasive cardiopulmonary hemodynamics prior to surgical myectomy or alcohol septal ablation suggests that pulmonary hypertension is often associated with left atrial hypertension, which may be due to dynamic left ventricular outflow tract obstruction and mitral regurgitation. However, we also observed in some patients an increase in pulmonary artery pressure without left atrial hypertension or other cardiopulmonary diseases commonly associated with pulmonary hypertension, raising the possibility that intrinsic pulmonary vascular remodeling was present in this obstructive HCM patient subgroup. (B) A significant difference in clinical response to septal reduction therapy with surgical myectomy or alcohol septal ablation (ASA) was not observed in patients with preprocedural pulmonary hypertension compared to patients with normal preprocedural mean pulmonary artery pressure (mPAP). LVOT, left ventricular outflow tract; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; HF, heart failure; NYHA, New York Heart Association; prC-PH, pre-capillary pulmonary hypertension; PAH, pulmonary arterial hypertension. The image of obstructive HCM was reproduced with permission from Nishimura RA, Ommen SR, Tajik AJ. Hypertrophic cardiomyopathy: A patient perspective. Circulation 2003;108:e133–e135.
On the other hand, we should emphasize that we found only an inconsistent relation between pulmonary artery pressure and LV outflow tract gradient or mitral regurgitation, allowing for the possibility that in some patients pulmonary hypertension represented intrinsic pulmonary vascular disease independent of mechanical left-sided obstruction and heart failure.15 In this regard, we identified a subset of patients (about 10%) with pulmonary hypertension associated with increased PVR, RV diameter, and DPG gradient, but normal PAWP. This scenario raises the possibility of co-existent pre-capillary pulmonary hypertension or PAH in some HCM patients. However, confirmation and further characterization of pulmonary vascular disease is needed in HCM patients, which will likely require histopathologic analysis of pulmonary tissue acquired from such patients. This is particularly the case since left atrial enlargement, borderline abnormal PAWP, and diuretic use were observed for HCM patients within this subgroup, suggesting that provocative maneuvers such as confrontational fluid challenge (which were not performed in this study) could unmask a contribution of left atrial hypertension to pulmonary hypertension in these patients.14
There are limitations to our study design that justify acknowledgement here. First, clinical care considerations did not allow us to routinely repeat cardiac catheterizations after surgery or alcohol ablation. The RHC was performed by multiple proceduralists over the span of the study period, likely with some minor variability in measurements. However, a blinded review of the RHC tracings was performed for the pre-capillary pulmonary hypertension patients, and this analysis confirmed the accuracy of the reported values. Additionally, the limited sample size of certain subgroups, such as subjects fulfilling hemodynamic criteria for PAH, prevented a more detailed characterization of this phenotype. Furthermore, we have resisted solely reporting estimates of pulmonary artery pressure with Doppler echocardiography postoperatively, given its relatively poor correlation with these measurements made directly at cardiac catheterization, as reported here and by other authors.16,17 Nevertheless, Mayo investigators using Doppler estimates both pre- and postoperatively showed that pulmonary hypertension was reversed by surgical myectomy in most HCM patients.3 Finally, we are lacking complete data on other possible contributors to pulmonary hypertension, such as the response of mPAP and PAWP to exercise or findings from additional diagnostic testing for the evaluation of pulmonary hypertension, which could be of value in achieving a better understanding of pathophysiology in our novel patient subset considered consistent with PAH.18,19
The number of HCM patients who underwent genetic testing in this cohort was too small to determine if specific sarcomere mutations were associated with pulmonary hypertension. However, given the large number of individual mutations now associated with HCM (>1,800), many of which are unique to one family (private), it is highly unlikely we would have identified a relationship between sarcomere mutation and pulmonary hypertension, similar to prior observations which have demonstrated no clear association between genotype and other aspects of the HCM phenotype (i.e., LV wall thickness, extent of fibrosis, obstruction) or outcome.20
In conclusion, pulmonary hypertension is common in obstructive HCM patients with severe heart failure. Unexpectedly, our data did not recognize elevated pulmonary pressures as a risk factor for adverse clinical outcome among obstructive HCM patients undergoing surgical myectomy (or alcohol septal ablation), nor do they suggest that such patients require intervention to normalize pulmonary pressures. Nevertheless, this analysis uncovered the distinct possibility of a new subset of HCM patients characterized by pulmonary vascular disease.
Supplementary Material
Clinical Summary.
Since the initial description of hypertrophic cardiomyopathy (HCM), left ventricular outflow tract obstruction has been considered a prominent determinant of heart failure. However, the clinical profile and contribution of pulmonary hypertension to limiting symptoms and management of obstructive HCM patients is incompletely understood. In this report, we analyzed invasive cardiopulmonary hemodynamic and echocardiography data from 162 consecutive patients with obstructive HCM referred for surgical septal myectomy. Pulmonary hypertension, defined as a mean pulmonary artery pressure ≥25 mmHg on cardiac catheterization, was present in a majority of HCM patients and was moderate or severe in 20%. Pulmonary artery pressure did not correlate with magnitude of left ventricular outflow tract gradient and was observed commonly in patients with normal pulmonary artery wedge pressure. In addition, in 11% of patients cardiopulmonary hemodynamics consistent with pre-capillary pulmonary hypertension or pulmonary arterial hypertension were present, suggesting that in a subgroup of patients, pulmonary hypertension may be a primary disease manifestation and not due to left heart disease. However, compared to patients with normal pulmonary artery pressure, pulmonary hypertension was not associated with a significant difference in clinical response or short-term outcome following surgical myectomy. Overall, our study shows that pulmonary hypertension is common and may be a novel contributor to heart failure in patients with obstructive HCM, but does not appear to influence surgical risk or clinical improvement from myectomy.
Acknowledgments
Sources of Funding: B.A.M.: (NIH) 1K08HL11207-01A1, American Heart Association (AHA 15GRNT25080016), Pulmonary Hypertension Association, Cardiovascular Medical Research and Education Fund (CMREF), and Klarman Foundation at Brigham and Women’s Hospital.
Footnotes
Conflict of Interest Disclosures: Dr. Bradley A. Maron receives funding from Gilead Sciences to research pulmonary hypertension. Dr. Preston IR receives funding from Actelion, Gilead, and United Therapeutics for clinical studies and consultancies.
References
- 1.Maron BJ, Rowin EJ, Casey SA, Maron MS. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality: Shaped by 50 years of clinical research and practice. JAMA Cardiol. 2016;1:98–105. doi: 10.1001/jamacardio.2015.0354. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Geske JB, Konecny T, Ommen SR, Nishimura RA, Sorajja P, Schaff HV, Ackerman MJ, Gersh BJ. Surgical myectomy improves pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Eur Heart J. 2014;35:2032–2039. doi: 10.1093/eurheartj/eht537. [DOI] [PubMed] [Google Scholar]
- 4.Ong KC, Geske JB, Hebl VB, Nishimura RA, Schaff HV, Ackerman MJ, Klarich KW, Siontis KC, Coutinho T, Dearani JA, Ommen SR, Gersh BJ. Pulmonary hypertension is associated with worse survival in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imag. 2016;17:604–610. doi: 10.1093/ehjci/jew024. [DOI] [PubMed] [Google Scholar]
- 5.Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Emande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron M. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 8.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. URL http://www.R-project.org. [Google Scholar]
- 9.Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, Kass DJ, Stephens T, Stanislawski MA, Swenson ER, Goldstein RH, Leopold JA, Zamanian RT, Elwing JM, Plomondon ME, Grunwald GK, Barón AE, Rumsfeld JS, Choudhary G. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: Insights from the VA-CART Program. Circulation. 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melacini P, Basso C, Angelini A, Calore C, Bobbo F, Tokajuk B, Bellini N, Smaniotto G, Zucchetto M, Iliceto S, Thiene G, Maron BJ. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. 2010;31:2111–2123. doi: 10.1093/eurheartj/ehq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maron BJ, Rowin EJ, Casey SA, Lesser JR, Garberich RF, McGriff DM, Maron MS. Hypertrophic cardiomyopathy in children, adolescents, and young adults associated with low cardiovascular mortality with contemporary management strategies. Circulation. 2016;133:62–73. doi: 10.1161/CIRCULATIONAHA.115.017633. [DOI] [PubMed] [Google Scholar]
- 12.Maron MS, Rowin EJ, Olivotto I, Casey SA, Arretini A, Tomberli B, Garberich RF, Link MS, Chan RH, Lesser JR, Maron BJ. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:1399–1409. doi: 10.1016/j.jacc.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Maron BA. Hemodynamics should be the primary approach to diagnosing, following, and managing pulmonary arterial hypertension. Can J Cardiol. 2015;31:515–520. doi: 10.1016/j.cjca.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galié N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 15.Rich S, Rabinovitch M. Diagnosis and treatment of secondary (non-category 1) pulmonary hypertension. Circulation. 2008;118:2190–2199. doi: 10.1161/CIRCULATIONAHA.107.723007. [DOI] [PubMed] [Google Scholar]
- 16.Farber HW, Foreman AJ, Miller DP, McGoon MD. Reveal registry: Correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56–64. doi: 10.1111/j.1751-7133.2010.00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Testani JM, John Sutton MG, Wiegers SE, Khera AV, Shannon RP, Kirkpatrick JN. Accuracy of noninvasively determined pulmonary artery systolic pressure. Am J Cardiol. 2010;105:1192–1197. doi: 10.1016/j.amjcard.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira RK, Waxman AB, Agarawal M, Badr Eslam R, Systrom DM. Pulmonary hemodynamics during recovery from maximum incremental cycling exercise. Eur Respir J. 2016;48:158–167. doi: 10.1183/13993003.00023-2016. [DOI] [PubMed] [Google Scholar]
- 20.Landstrom AP, Ackerman MJ. Mutation type is not clinically useful in predicting prognosis in hypertrophic cardiomyopathy. Circulation. 2010;122:2441–2449. doi: 10.1161/CIRCULATIONAHA.110.954446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


