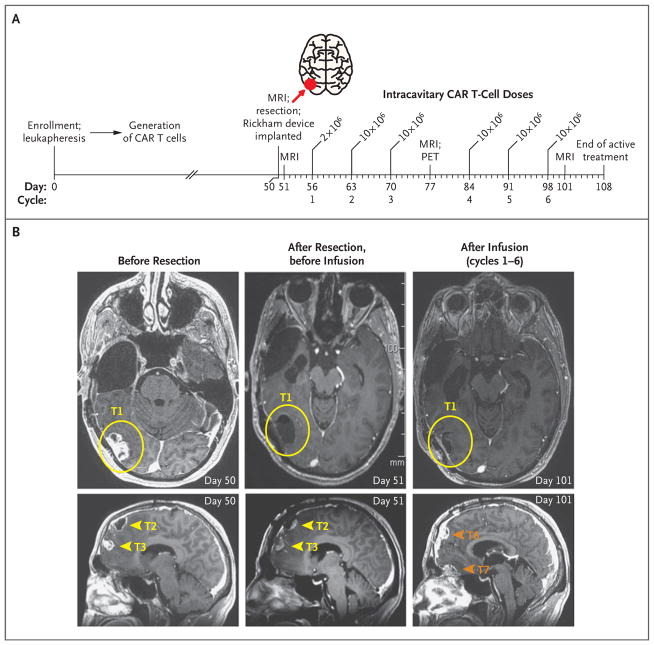
Figure 1. Local Tumor Control after Intracavitary Delivery of IL13BBζ–Chimeric Antigen Receptor (CAR) T Cells.
Panel A shows an overview of the intracavitary administration of six cycles of IL13BBζ–CAR T cells according to dose schedule 1 (see Table S1 in the Supplementary Appendix). CAR T cells were delivered through a Rickham catheter device into the right temporal–occipital region (tumor 1; red arrow) from day 56 to day 98 after enrollment, with 1 week of rest after cycles 3 and 6 for evaluation of safety and disease. MRI denotes magnetic resonance imaging, and PET positron-emission tomography. Panel B shows axial MRI (T1-weighted with gadolinium enhancement) of the brain highlighting the site of the resected tumor at which the catheter was placed for delivery of CAR T cells (tumor 1 [T1]; yellow circles), as well as the resected-only tumor sites in the frontal lobe (tumors 2 and 3 [T2 and T3]; yellow arrowheads) and the sites of tumors that developed during the intracavitary treatment period (tumors 6 and 7 [T6 and T7]; orange arrowheads). The CAR T-cell injection site (T1) remained stable without evidence of disease recurrence, whereas other disease foci, including T6 and T7, which were adjacent to resected T2 and T3 but distant from the CAR T-cell injection site, continued to progress.