Abstract
Approach-avoidance conflict (AAC) refers to situations associated with both rewarding and threatening outcomes. The AAC task was developed to measure AAC decision-making. Approach behavior during this task has been linked to self-reported anxiety sensitivity and has elicited anterior cingulate, insula, caudate and right dorsolateral prefrontal cortex (dlPFC) activity, with right lateral PFC tracking the extent of approach behavior. Guided by these results, we used excitatory transcranial direct current stimulation (tDCS) to demonstrate the causal involvement of right dlPFC in AAC decision-making. Participants received anodal tDCS at 1.5mA over either left or right dlPFC or sham stimulation, while performing the AAC task and a control short-term memory task. Analyses of variance (ANOVA) revealed that for individuals with high anxiety sensitivity excitatory right (but not left or sham) dlPFC stimulation elicited measurable decreases in approach behavior during conflict. Excitatory left (but not right or sham) dlPFC simulation improved performance on the control task. These results support a possible asymmetry between the contributions of right and left dlPFC to AAC resolution during emotional decision-making. Increased activity in right dlPFC may contribute to anxiety-related symptoms and, as such, serve as a neurobehavioral target of anxiolytic treatments aiming to decrease avoidance behavior.
Keywords: anxiety, approach-avoidance conflict, decision making, transcranial direct current stimulation, dorsolateral prefrontal cortex
Frequently in daily life, we are faced with situations in which the decision to move forward with a particular behavior is concurrently associated with both a rewarding and a threatening outcome (e.g. interviewing for a new job can be stressful, but also rewarding if one is successful). Decision-making under approach-avoidance conflict (AAC) conditions is uniquely challenging because it requires the integration of information regarding the value of potential rewards and cost of potential punishments, as well as the likelihood and magnitude of each outcome and its importance for the integrity of the individual (Quartz, 2009; Aupperle and Paulus, 2010; Aupperle et al., 2015). Understanding AAC decision-making may have important consequences for the conceptualization and treatment of anxiety disorders (Stein and Paulus, 2009; Aupperle and Paulus, 2010), which typically entail avoidance of short-term emotional discomfort, even at the cost of substantial long-term rewards (e.g. Foa and Kozak, 1986; Barlow, 2002).
Animal models of anxiety have employed AAC paradigms that typically establish a conflict between approaching a reward (e.g. water or food) and avoiding a punishment (e.g. receiving a mild electric shock). The anxiolytic potential of a pharmacologic agent is often assessed by determining its ability to increase approach behavior during animal conflict paradigms (see Millan, 2003; Millan and Brocco, 2003). Approach behaviors during such conflict conditions are significantly increased following lesions to the amygdala (e.g. Kopchia et al., 1992) and medial prefrontal cortex (PFC; e.g. Resstel et al. 2008). Neuroimaging studies with human participants have further associated activity in the amygdala, insula, medial PFC, and anterior cingulate cortex (ACC) with emotion regulation, especially in the presence of threatening stimuli (Davis and Whalen, 2001; Ochsner and Gross, 2005; Craig, 2009, 2011; Shin and Libertzon, 2010; Shackman et al., 2011). Moreover, a network of PFC regions including the ACC, orbitofrontal cortex (OFC), and dorsolateral PFC (dlPFC), in conjunction with the amygdala and the ventral striatum, have been implicated in approach-avoidance learning and trait motivations, with the right PFC typically associated with avoidance motivations (e.g. Spielberg et al., 2012) and the ventral striatum, medial OFC, and left PFC typically associated with approach motivations (e.g. Simon et al., 2010; Spielberg et al., 2012). Particularly under conditions of emotional conflict, the right ACC and ventral striatum appear to be modulated by punishment (Talmi et al., 2009), whereas left rostral ACC can adaptively resolve emotional conflict by top-down inhibition of the amygdala (Etkin et al., 2006).
Despite a substantial body of research examining the involvement of these fronto-striatal and amygdala circuits to approach-avoidance learning, much less work has focused on the neural mechanisms underlying the process of decision making during conflict. Recently, Aupperle et al. (2011) developed the AAC paradigm that quantifies decision-making using a continuous measure of approach-avoidance behavior during situations that involve various degrees of conflict. As the punishments involved in the task are affective in nature (combination of negative affective images and sounds), it provides a model of emotional decision-making in real-life situations that can have implications for the understanding and treatment of anxiety disorders. The extent of approach behavior on this task has been related to either self-reported anxiety sensitivity or behavioral activation (Aupperle et al., 2011). Functional magnetic resonance imaging (fMRI) measurements during the AAC task have shown that conflict (relative to non-conflict) decisions are associated with increased recruitment of ACC, insula, caudate and right dlPFC regions (Aupperle et al., 2015). Moreover, right lateral PFC activation (BA 6 and BA 10/46) on a trial-by-trial basis was related to less approach behavior during conflict, and activity in the right dlPFC (BA 9) and caudate were modulated by the level of reward offered during conflict. This pattern of results supports a possible asymmetry between contributions of the right and left lateral PFC to AAC resolution during emotional decision-making, according to which increased activity in the right PFC may lead to less approach behavior during conflict and, as such, underlie certain anxiety-related behaviors. However, right dlPFC activity during the AAC task may not only lead to a higher propensity to avoid conflicting outcomes, but may also guide decision making by augmenting valuation of outcome and reinforcement information (Mitchell, 2011).
The aim of this study was to amplify past neuroimaging findings and examine the causal relationship between right dlPFC activation and approach behavior during AAC decision making. Specifically, we used anodal transcranial direct current stimulation (tDCS) to enhance activity in right dlPFC; tDCS is a noninvasive technique that involves the application of small direct currents (typically 1–2mA) to the scalp for a few minutes through two surface electrodes, which can modulate cortical excitability in the underlying brain region. During and immediately after application, anodal tDCS stimulation increases cortical excitability at the stimulation site through subthreshold neuron soma depolarization, whereas cathodal tDCS stimulation decreases cortical excitability due to neuron soma hyperpolarization (Nitsche et al., 2008; Stagg and Nitsche, 2011). As such, tDCS has been increasingly used in various domains within clinical neuroscience to establish relationships between activity in a particular brain region and the expression and treatment of psychopathological symptomatology for different psychiatric disorders, including schizophrenia (Brunelin et al., 2013), major depression (Brunoni et al. 2013), obsessive-compulsive disorder (Volpato et al., 2013) and generalized anxiety disorder (GAD) (Shiozawa et al., 2014).
Our objective in this study was to use anodal tDCS to demonstrate causal involvement of right dlPFC to AAC decision-making. Based on previous research and the neuroimaging results by Aupperle et al. (2015), we hypothesized that application of anodal tDCS over the right dlPFC would decrease approach behavior in the AAC task. Given the link between anxiety and avoidance behavior, as well as our past results linking anxiety sensitivity to AAC approach behavior (Aupperle et al., 2011), we anticipated the effect of excitatory right dlPFC stimulation to be more pronounced among high anxiety-sensitive participants. To examine the regional specificity of this effect, we also included a group of participants who received anodal stimulation over the homologous region in the left hemisphere. Although past research (e.g. Sutton and Davidson, 1997; Berkman and Lieberman, 2010) has linked left dlPFC activity to approach behavior or motivations, our past neuroimaging findings did not reveal significant left dlPFC contributions to behavior on the AAC task, specifically designed to measure approach-avoidance decision making. We therefore did not anticipate that anodal tDCS over left dlPFC would influence AAC task approach behavior. Further, to control for the likelihood that anodal tDCS would influence performance regardless of the nature of the task (e.g. due to differences in arousal), we included as a control task the Forward Digit Span, a short-term memory task, which is unrelated to right dlPFC function, but could be influenced by changes in left dlPFC activity (see Owen et al., 2000).
Methods
Participants
Sixty-three (n = 63) college students (mean age = 19 years, 52% male, 78% Caucasian) participated in the study for class credit, after providing written informed consent. Participants were excluded from the study if they reported left-handedness, current pregnancy, or a history of a mood disorder, seizure disorder, or head injury. The study was approved by the University of Kansas Institutional Review Board.
Materials
Demographic information. A brief self-report questionnaire obtained information on the participants’ native language, gender, age, race, education level, and any history of psychopathological disorders, phobias or abuse.
Anxiety sensitivity measures. The Anxiety Sensitivity Index (ASI, Reiss et al., 1986) is a 16-item measure in which participants indicate, on a scale from 0 (Very little) to 4 (Very much), the extent of their concern about potential negative consequences of anxiety-related feelings. The ASI has good validity and reliability (α = 0.88; Peterson and Heilbronner, 1987).
Behavioral approach and inhibition measures. The Behavioral Inhibition System-Behavioral Approach System (BIS-BAS; Carver and White, 1994) is a 24-item measure that assesses, on a scale from 1 (very true for me) to 4 (very false for me), how motivated an individual is to move away from something unpleasant or move towards something desired. The BIS-BAS has four subscales (BAS Drive, BAS Reward Responsiveness, BAS Fun Seeking and BIS) and strong validity and reliability (α = 0.70–0.83; Jorm et al., 1998).
State positive and negative affect measures.The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) is a 20-item scale that assesses positive and negative affect. Participants respond, on a scale from 1 (very little) to 5 (extremely), the extent to which a series of adjectives describes how they are feeling in the moment. The validity and reliability of the PANAS have been established (α = 0.84–0.90; Watson et al., 1988).
Forward digit span. The forward digit span (FDS) task was adapted from the Wechsler Adult Intelligence Scale—Fourth Edition (Pearson Education, Inc.) and it is used to measure one’s ability to maintain information in phonological short-term memory. Participants are read a string of numbers and are asked to repeat these numbers back in the same order. The task begins with two-digit number strings and progresses to nine-digit strings (two trials per string length). Participants discontinue after responding incorrectly to both trials of a given string length.
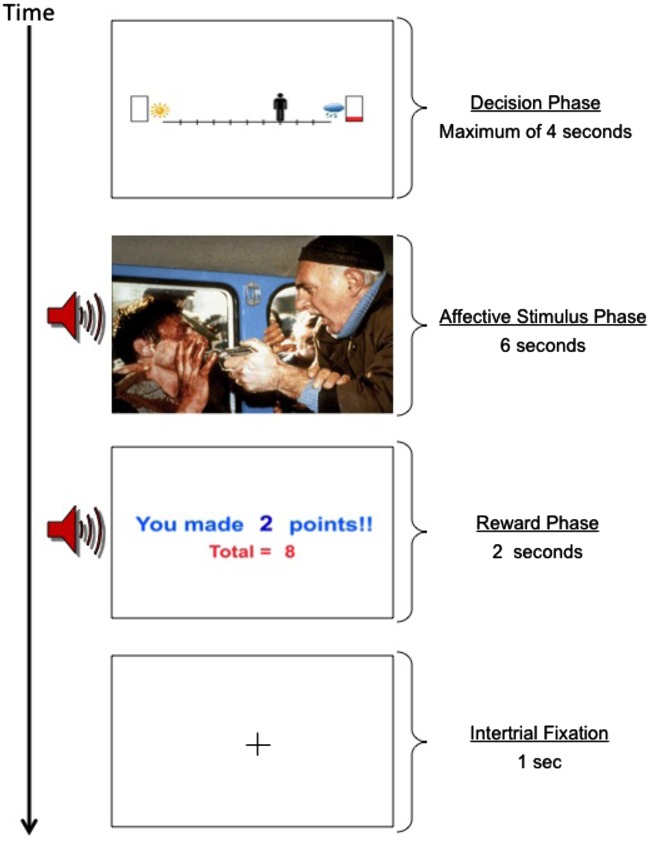
AAC task. We used a version of the AAC task adapted from past work (Aupperle et al., 2011, 2014). For each trial, participants are first shown a runway with pictures on each side to represent two potential outcomes (Figure 1). Each potential outcome includes an affective stimulus and a certain level of reward points. A picture of a sun indicates a positively valenced affective stimulus, whereas a cloud indicates a negatively valenced affective stimulus. The number of reward points to be received if a given outcome were to occur is indicated by the amount of red in the rectangles. Reward points are only offered with one of the outcomes. The participants use the arrow buttons to move the avatar on the runway to indicate their relative preference for potential outcomes, having 4 s to respond. The location of the avatar at the end of the decision phase corresponds to the probability of the two outcomes occurring. For instance, if the avatar is in the middle of the runway, there is a 50% chance of each outcome; if the avatar is all the way to one side, there is a 90% chance of the nearest outcome and a 10% chance of the farther outcome. Therefore, participants control the likelihood of the outcomes but are unable to determine with absolute certainty which outcome will occur. The starting position of the avatar can influence both initial response time and end avatar position. Thus, the starting position of the avatar is counterbalanced across trials (for each condition type) to control for these effects. At the end of the task, a screen appears displaying total points received and a corresponding award ribbon. The points do not translate into monetary reward, and thus, participants are playing for points only. However, in the beginning of the task, participants are told that their performance will be ranked against past participants, creating a higher desire for reward points.
Fig. 1.
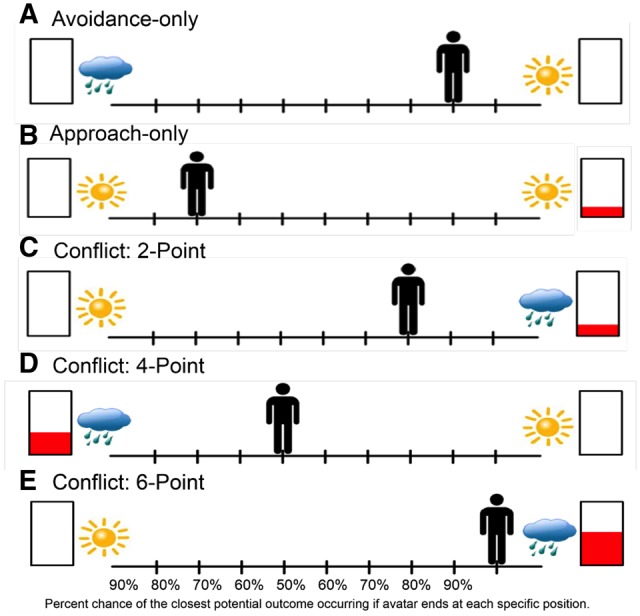
Example of the five AAC trial types.
The ACC task is programmed using Adobe Flash Professional CS5. The affective stimuli include image and sound combinations collected from the International Affective Picture System (Lang et al., 2008), International Affective Digitized Sounds (Bradley and Lang, 1999) and other freely available audio files. The reward includes 0, 2, 4 or 6 points presented along with a trumpet sound. There are three trial types (see Figure 1): (i) ‘Avoidance-only’ (AV), in which 0 points are offered for both outcomes and thus, there is no explicit motivation to approach the negative affective outcome; (ii) ‘Approach-only’ (APP), in which 2 versus 0 points are offered but with positive affective stimuli associated with both outcomes. For these conditions, there is no explicit motivation to ‘not’ approach the rewarded outcome. (iii) Three levels of ‘Conflict’ in which 2 (CONF2), 4 (CONF4) or 6 (CONF6) points are offered for the outcome involving a negative affective stimulus whereas 0 points are offered for the outcome involving a positive affective stimulus. These conditions are designed to produce AAC, as the same behavior is associated with both reward and punishment. The participants have no way of knowing the valence or arousal level of the potential negative affective outcome and that outcome is not proportionate to the level of reward points. Thus, the increasing reward level is meant to increase reward motivation to approach the negative affective stimulus. The task was shortened from its original version to stay within the maximum tDCS stimulation duration of 20 min. The current task included a total of 70 trials, with 14 of each trial type (AV, APP and three levels of conflict). The timing of each AAC trial is displayed in Figure 2.
Fig. 2.
The timing of AAC task stimuli.
The main dependent variables for the AAC task were as follows: (i) Mean approach behavior across the three reward conflict trials, or the avatar’s end position on the runway in relation to the negative affective outcome, ranging from −4 (avoidance all the way away from the negative affective stimulus and reward) to +4 (approach all the way towards the negative affective stimulus and reward). (ii) Approach behavior contingent on the number of reward points (change from 2–4 to 6 points). For the purposes of this study, approach behavior was calculated for both non-conflict and conflict trials.
Design and procedure
Participants were randomly assigned to one of three conditions: (i) anodal stimulation over the right dlPFC (n = 21; 65% males), or (ii) anodal stimulation over the left dlPFC (n = 21; 62% males) or (ii) sham stimulation (n = 21; 29% males), which were blind to the participant. Prior to stimulation, participants completed a screening questionnaire to ensure they were eligible (see above criteria) and females were also administered a pregnancy test. If eligible, participants completed the PANAS, and then were given three practice trials of the AAC task to ensure full understanding of the task. After the practice task, tDCS stimulation was initiated.
tDCS parameters. TDCS was administered through two 5 × 5 cm electrodes covered in saline solution-soaked sponges. The site of stimulation was determined through a BraiNet 10/20 placement cap (see biomedical.com) and was noted with a marker on the participant’s scalp. Guided by the neuroimaging results of Aupperle et al. (2015), the anode was placed either over area F4 or F3 on the 10/20 system for stimulation of the right or left dlPFC respectively (DaSilva et al., 2011; Homan et al., 1987). A reference cathode electrode was placed over the contralateral mastoid. tDCS stimulation was applied using a DC-Stimulator for tDCS (NeuroConn, GmbH, http://www.neuroconn.de/dc-stimulator_en/) at 1.5mA for a maximum of 20 min (including 10 seconds ramp-up and 10 s ramp-down time). These parameters are within safety limits established from prior work in humans (e.g. Nitsche et al., 2003; Bikson et al., 2009; Tadini et al., 2011).
During the two stimulation conditions, stimulation started for 180 s prior to the initiation of the AAC or FDS task to ensure maximum excitability changes following tDCS (see Nitsche and Paulus, 2000). During the sham condition stimulation began but unbeknownst to the participants was terminated after 90 s. The placement of the anode for the sham condition
either over F3 or over F4) and the order of the behavioral tasks (i.e.FDS or AAC) were counterbalanced across participants. Both tasks were completed concurrently with stimulation. Upon completion of these tasks, participants were administered the PANAS, followed by the remaining self-report questionnaires.
Data preparation and analysis
For the PANAS we calculated negative affect change by subtracting the individual’s negative affect score from the PANAS completed prior to the AAC task from their score after the AAC task (i.e. negative affect post-AAC task minus negative affect pre-AAC task); a similar procedure was followed for positive affect. For the FDS control task we calculated the total number of correct responses (out of a possible 16). For the AAC task, we first calculated the mean approach behavior (range = −4 [all the way away from reward points] to 4 [all the way towards the reward points]) across the 2-, 4- and 6-point conflict trials, with higher scores indicating higher approach behavior. Approach behavior contingent upon reward points was calculated through the following equation: (6–4-point mean approach score) + (4–2-point mean approach score). Higher scores on this measure indicate that conflict behavior was more influenced by the level of reward points offered. ANOVA were used to examine differences between stimulation conditions (rdlPFC, ldlPFC, sham) in regards to self-report measures and affect change (post-pre AAC task), and FDS performance. For the AAC task, we hypothesized that any potential effects of stimulation would be more prominent for high- relative to low-anxiety individuals. Accordingly, we divided participants across conditions into high- and low-anxiety based on the median score on the ASI. ANOVA were then used to examine main interaction effects of stimulation condition and ASI group on AAC task behavior (approach behavior). Results were considered significant at P < 0.05. We used Tukey’s Honestly Significant Difference (HSD) tests for all post hoc comparisons.
Results
Individual differences measures
There were no effects of task order, thus data were combined across order conditions. One participant in the right dLPFC excitation condition was identified as a multivariate outlier (according to Mahalanobis distance) across the self-report and FDS measures, and was excluded from all further analyses. ANOVA revealed no a priori differences among the three stimulation conditions on any aspect of the ASI and behavioral approach and inhibition (BIS-BAS) measures (Ps ranged from 0.47 to 0.98; see Table 1); thus, there were no a priori systematic differences on these factors among stimulation conditions that would confound tDCS effects on the AAC task.
Table 1.
Mean anxiety and affect measures by condition
| rdlPFC | ldlPFC | Sham | |
|---|---|---|---|
| ASI | 21.85 (9.74) | 20.09 (10.46) | 22.00 (15.63) |
| Behavioral Approach Drive | 8.55 (2.14) | 8.00 (2.28) | 8.33 (2.13) |
| Behavioral Inhibition | 15.10 (2.69) | 14.38 (3.49) | 15.62 (4.26) |
| Behavioral Approach Reward Responsiveness | 8.00 (2.00) | 7.67 (1.74) | 8.00 (1.64) |
| Behavioral Approach Fun Seeking | 8.00 (1.97) | 7.76 (1.94) | 7.29 (1.75) |
| Negative affect change | 1.60 (2.98) | 0.29 (5.22) | −1.24 (5.56) |
| Positive affect change | −0.55 (4.93) | −1.57 (4.51) | 2.81 (3.67) |
| Average approach behavior across 2, 4, 6 point conflict conditions | 2.27 (2.37) | 2.33 (2.02) | 2.34 (1.76) |
| Average approach behavior contingent upon reward points | 0.78 (2.35) | 1.33 (1.77) | 0.76 (2.31) |
SD in parenthesis.
Affect
We examined possible differences in affect following the AAC task (see Table 1). Across stimulation conditions, there was a significant decrease in positive affect from prior to (mean = 32.19, SD = 7.51) relative to after the task (mean = 30.53, SD = 8.14) the AAC task [F(1, 61) = 8.77, P = 0.004, ηp2 = 0.13]; the difference in negative affect prior to (mean = 16.84, SD = 5.87) relative to after (mean = 17.03, SD = 6.71) the task was not significant [F(1, 61) = 0.10, P = 0.75, ηp2 = 0.002]. ANOVA revealed no significant differences in negative [(2, 59) = 1.84, P = 0.17, ηp2 = 0.06] or positive [F(2, 59) = 1.36, P = 0.26, ηp2 = 0.04] affect change for the stimulation conditions; Tukey’s HSD post hoc tests did not reveal any statistically significant pairwise comparisons (Ps ranged from 0.13 to 0.73).
FDS
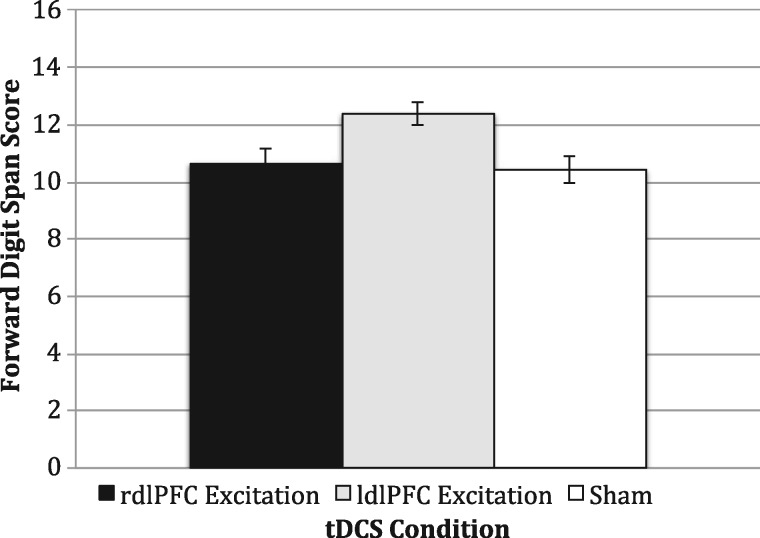
ANOVA results showed that FDS performance was disproportionally affected by tDCS across conditions [F(2, 59) = 5.45, P = 0.007, ηp2 = 0.16; see Figure 3]. In line with our hypothesis, post hoc pairwise comparisons (Tukey’s HSD tests) showed that participants who received excitatory left dlPFC stimulation outperformed participants who received excitatory right dlPFC (P = 0.03, Cohen’s d = 0.84) or sham (P = 0.01, Cohen’s d = 0.99) stimulation, who did not differ from each other (P = 0.94, Cohen’s d = 0.09). Thus, left, but not right, dlPFC excitation significantly influenced performance on this task.
Fig. 3.
Effects of stimulation on the FDS task. rdlPFC, right dorsolateral prefrontal cortex; ldlPFC, left dorsolateral prefrontal cortex; tDCS, transcranial direct current stimulation. Error bars indicate the SEMs.
AAC task
We first examined the main effects of task on approach behavior across stimulation conditions. As predicted, participants exhibited more approach behavior for the approach-only trials (mean = 3.69, SD = 0.87) than for the avoid-only trials [mean = −3.25, SD = 1.28; F(1, 61) = 736.63, P < 0.001, ηp2 = 0.92]. Moreover, there was an increase in approach behavior from the 2-point (mean = 1.99, SD = 2.34), to the 4-point (mean = 2.26, SD = 2.20), to the 6-point (mean = 2.69, SD = 1.83) conflict trials [F(2, 122) = 10.82, P <0.001, ηp2 = 0.15]. This confirms that participants understood the task and, generally, performed similarly to that reported in previous studies using the AAC task.
According to the median ASI split, the Low ASI group ASI scores ranged from 4 to 20 (mean = 12.81, SD = 4.96); the High ASI group ASI scores ranged from 21 to 67 (mean = 30.97, SD = 10.45; for clinical comparisons, the average ASI score for individuals diagnosed with anxiety and related disorders is 45, Rodriguez et al., 2004). In the Low ASI group there were 12 participants (11 males) in the rdlPFC condition; 9 (5 males) in the ldlPFC condition, and 12 (4 males) in the sham condition. In the High ASI group there were 8 participants (2 males) in the rdlPFC condition; 12 (8 males) in the ldlPFC condition, and 9 (2 males) in the sham condition. Low ASI participants differed from High ASI participants in anxiety score in the rdlPFC [t(19) = 4.88, P < 0.001], ldlPFC [t(18) = 6.94, P < 0.001], and sham [t(19) = 5.47, P < 0.001] conditions. Low and High ASI participants did not differ in their BIS-BAS or PANAS scores in the rdlPFC, ldlPFC or sham conditions (all Ps > 0.10). Given that previous work has reported gender effects on the AAC task, we first examined ASI and gender interaction effects on AAC approach behavior. For approach behavior on the AAC averaged across 2, 4 and 6 reward points there were no significant main effects of gender F[1, 58] = 1.86, P = 0.17, ηp2 = 0.03) or ASI condition [F(1, 58) = 0.09, P = 0.77, ηp2 = 0.001], and no gender × ASI condition interaction [F(1, 58) = 0.13, P = 0.72, ηp2 = 0.002]1.
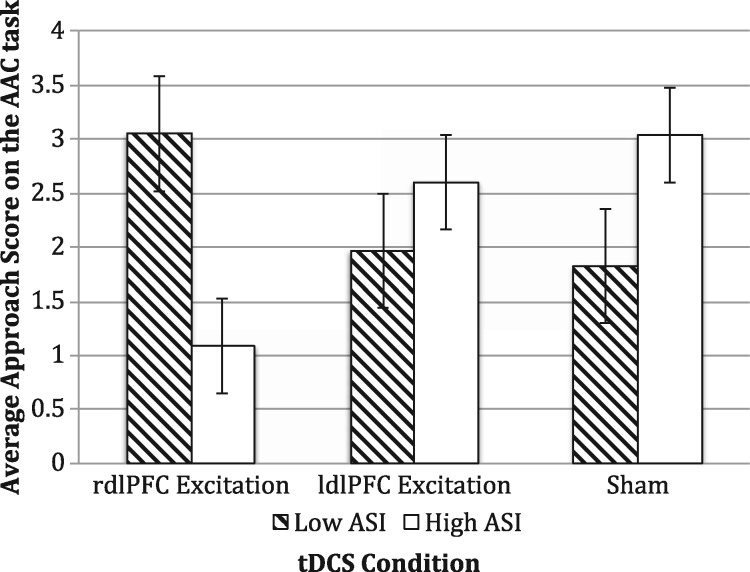
ANOVA revealed no main effects of stimulation [F(5, 56) = 0.17, P = 0.85, ηp2 = 0.006] or ASI [F(5, 56) = 0.005, P = 0.94, ηp2 = 0.00] condition; however, in line with our predictions, there was a significant tDCS condition × ASI condition interaction [F(5, 56) = 3.55, P = 0.036, ηp2 = 0.11], according to which participants with high anxiety sensitivity who underwent excitatory stimulation over right dlPFC exhibited lower approach behavior during conflict (see Figure 4). Tukey’s HSD post hoc pairwise tests did not reveal any statistically significant differences between stimulation conditions (Ps > 0.67). Main and interaction effects of stimulation condition and ASI on AAC approach behavior contingent upon reward points were not significant.
Fig. 4.
Effects of stimulation on the AAC task by ASI condition. rdlPFC, right dorsolateral prefrontal cortex; ldlPFC, left dorsolateral prefrontal cortex; tDCS, transcranial direct current stimulation; AAC, Approach Avoidance Conflict; ASI, Anxiety Sensitivity Index. Error bars indicate the SEMs.
Discussion
AAC has been consistently associated in past studies with increased activity in right dlPFC. Guided by our past neuroimaging findings (Aupperle et al., 2015), here, we used anodal tDCS to demonstrate that increased right dlPFC activity may lead to decreases in approach behavior during AAC. We predicted that participants undergoing excitatory stimulation over right (but not left or sham) dlPFC would show decreased approach behavior, an effect we anticipated to be stronger among participants with high anxiety sensitivity. Our results partially supported these predictions: Participants high on anxiety sensitivity showed significantly limited approach behavior under excitatory right (but not left or sham) tDCS over dlPFC. This effect was not attributed to transient differences in mood or a priori systematic differences in anxiety among stimulation conditions. Consistent with our predictions, excitation over the left (but not right or sham) dlPFC enhanced performance on the control FDS task.
These results are consistent with findings of Shiozawa et al. (2014) who demonstrated that 15 consecutive daily sessions of cathodal (inhibitory) tDCS at 2.0 mA over right dlPFC significantly reduced anxiety symptoms in a patient with GAD. Similarly, fMRI-guided, low-frequency (i.e. inhibitory) repetitive transcranial magnetic stimulation (rTMS) over dlPFC has been used effectively in the treatment of GAD (Bystritsky et al., 2008; see also Pallanti et al., 2012 for a review). Our results used anodal tDCS over right dlPFC to support and extend past research by demonstrating the potentially causal role of over-excitation of right dlPFC for affective decision-making in anxiety disorders. Future research investigating cathodal tDCS of the right dlPFC on AAC decision-making could help to elucidate mechanisms for the above clinical reports. In this study, we focused specifically on anxiety sensitivity (which may relate more to fear of bodily sensations) due to our previous findings with the AAC task. However, further research is needed to determine whether current results are specific to anxiety sensitivity or more general to other aspects of normative and clinical anxiety.
Although past research has typically associated left PFC with approach motivations (e.g. Berkman and Lieberman, 2010; Simon et al., 2010; Spielberg et al., 2012; see also Sutton and Davidson, 1997) our results did not reveal any significant influence of left dlPFC excitation on AAC task behavior. Relative to other approach-avoidance paradigms (e.g. Berkman and Lieberman, 2010), our AAC task was specifically designed to capture AAC ‘decision-making’, which has not been associated with significant left dlPFC contributions (Aupperle et al., 2015). The lack of left dlPFC excitation effects on approach behavior is, thus, consistent with our previous neuroimaging findings. It is possible that differences in task stimuli could alter the relative importance of right versus left dlPFC in contributing to behavior; for example, depending on the saliency or primacy of threats and rewards (i.e. affective stimuli, food pictures, vs monetary loss or reward). Related to this, we also did not find effects for right dlPFC excitation on conflict behavior contingent upon increasing reward points. It is possible that right dlPFC excitation does not impacting reward-dependent changes in behavior as much as it does conflict behavior in general, which is partially driven by avoidance motivations.
Notably, even in the right dlPFC stimulation condition, individuals with higher anxiety were, on average, placing the avatar at a position of approximately one (with −4 being all the way away from the conflict outcome; and +4 being all the way towards that conflict outcome). Thus, these participants were not avoiding per se, but experiencing greater conflict, or uncertainty, in how to respond. This may reflect the fact that participants were all healthy controls with nonclinical levels of anxiety. However, it also raises the question of whether right dlPFC over-activation increases the propensity to avoid conflict outcomes or whether it more generally interrupts optimal balancing of potential outcomes to resolve conflict decisions. Although the former would be consistent with previous research suggesting dlPFC stimulation may reduce risk-taking behavior (Fecteau et al., 2007), the latter would be consistent with the proposed role of the dlPFC in guiding final action by augmenting valuation of outcome and reinforcement information (Mitchell, 2011). It is possible that either under- or over- activation of the right dlPFC could have a negative impact on conflict decision-making. In addition, the reported results seemed to not only relate to decreased approach behavior for those reporting high ASI, but also increased approach behavior for those reporting low ASI, in response to right dlPFC stimulation. Thus, the functional impact of right dlPFC stimulation may depend on the baseline anxiety level of the individual. These are questions that could be addressed by future research.
Our particular electrode montage was exclusive to dlPFC with the reference electrode away from the cortex, which allowed us to examine the specific effects of dlPFC excitation on this task. Nevertheless, we cannot exclude the possibility that excitatory effects over this region might have downstream, secondary influences on other cortical and subcortical structures, including the medial PFC, amygdala or insula. Visualization of the path of the electric current for our particular montage with electrical field modeling (as implemented in the Enobio tool, Neuroelectrics, Barcelona, Spain) has allowed us to confirm that excitation over right or left dlPFC does not carry to the homologue region of the other hemisphere. However, it is likely that anodal stimulation may have induced the observed effects due to secondary influences on other subcortical structures. Specifically, our previous neuroimaging research would suggest that other areas of the lateral PFC (i.e. BA 6, 10) might be involved in processing and responding to AAC. Future research employing concurrent tDCS and neuroimaging may be in position to delineate the precise effects of neurostimulation for the function of cortical and subcortical networks in space and time.
In previous work with the AAC task, we have demonstrated potential gender effects, with males exhibiting greater approach behavior (consistent with animal work) and being influenced more by anxiety sensitivity (females being influenced more by self-reported behavioral activation). Notably, in the current sample gender was not equal across the ASI groups (with more females in the high ASI group). Although gender did not seem to be driving differences in approach behavior in this study, we did not have the sample size to delineate potential differences in dlPFC stimulation by gender, which would be an important aim for future research. Larger studies, using continuous (rather than dichotomized) anxiety measures, could also help in clarifying the extent to which anxiety moderates the relationship between dlPFC stimulation and performance on the AAC task.
Overall, our results demonstrate that an over-excitation of right dlPFC in healthy adults with high sensitivity to anxiety can lead to decreases in approach behavior during affective decision making. As such, they contribute to the growing body of literature detailing the contribution of this region to anxiety disorders and point to the potential of tDCS interventions for the treatment of large patient groups.
Funding
Evangelia G. Chrysikou, Ph.D. receives funding from the Brain and Behavior Research Foundation under Award Number 23980. Robin Aupperle, Ph.D. receives funding from the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH108707.
Conflict of interest: None declared.
Footnotes
1 We examined interactions with gender especially in the context of this gender distribution; controlling for gender did not make a difference in our analysis. This result is possibly due to the lack of statistical power that did not permit a full examination of gender effects. On the other hand, the lack of a significant main effect of gender or a significant ASI by gender interaction points to the effectiveness of our manipulation independently of any gender influence.
References
- Aupperle R.L., Melrose A.J., Francisco A., Paulus M.P., Stein M.B. (2015). Neural substrates of approach-avoidance conflict decision making. Human Brain Mapping, 36, 449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Paulus M.P. (2010). Neural systems underlying approach and avoidance in anxiety disorders. Dialogues in Clinical Neuroscience, 12, 305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Sullivan S., Melrose A.J., Paulus M.P., Stein M.B. (2011). A reverse translational approach to quantify approach-avoidance conflict in humans. Behavioural Brain Research, 225, 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D.H. (2002). Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. New York: Guilford Press. [Google Scholar]
- Berkman E.T., Lieberman M.D. (2010). Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience, 22, 1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., Datta A., Elwassif M. (2009). Establishing safety limits for transcranial direct current stimulation. Clinical Neurophysiology, 120, 1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. (1999). International affective digitized sounds (IADS): Stimuli, instruction manual, and affective ratings. Techical Report No. B-2. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida.
- Brunelin J., Mondino M., Gassab L., et al. (2012). Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. American Journal of Psychiatry, 169, 719–24. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Valiengo L., Baccaro A., et al. (2013). The sertraline vs electrical current therapy for treating depression clinical study. JAMA Psychiatry, 70, 383–91. doi:10.1001/2013.jamapsychiatry.32 [DOI] [PubMed] [Google Scholar]
- Bystritsky A., Kaplan J.T., Feusner J.D., et al. (2008). A preliminary study of fMRI-guided rTMS on the treatment of generalized anxiety disorder. Journal of Clinical Psychiatry, 69, 1092–8. [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–33. [Google Scholar]
- Craig A.D. (2009). How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- DaSilva A.F., Volz M.S., Bikson M., Fregni F. (2011). Electrode positioning and montage in transcranial direct current stimulation. Journal of Visualized Experiments, 51, e2744. doi:10.3791/2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–82. [DOI] [PubMed] [Google Scholar]
- Fecteau S., Pascual-Leone, A., Zald, D.H., et al. (2007). Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. The Journal of Neuroscience, 27(23), 6212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E.B., Kozak M.J. (1986). Emotional processing of fear: Exposure to corrective information. Psychological Bulletin, 99, 20–35. [PubMed] [Google Scholar]
- Homan R.W., Herman J., Purdy P. (1987). Cerebral location of international 10-20 system electrode placement. Electroencephalography and Clinical Neurophysiology, 66, 376–82. [DOI] [PubMed] [Google Scholar]
- Jorm A.F., Christensen H., Henderson A.S., Jacomb P.A., Korten A.E., Rodgers B. (1998). Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity, and norms in a large community sample. Personality and Individual Differences, 26, 49–58. [Google Scholar]
- Kopchia K.L., Altman H.J., Commissaris R.L. (1992). Effects of lesions of the central nucleus of the amygdala on anxiety-like behaviors in the rat. Pharmacology, Biochemistry, and Behavior, 43, 453–61. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008). International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual, Technical Report a-8. Gainesville, FL: University of Florida. [Google Scholar]
- Millan M.J. (2003). The neurobiology and control of anxious states. Progress in Neurobiology, 70, 83–244. [DOI] [PubMed] [Google Scholar]
- Millan M.J., Brocco M. (2003). The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. European Journal of Pharmacology, 463, 67–96. [DOI] [PubMed] [Google Scholar]
- Mitchell D.G.V. (2011). The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behavioural Brain Research, 217.1, 215–31. [DOI] [PubMed] [Google Scholar]
- Nitsche M., Cohen L., Wassermann E., et al. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1, 206–23. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Liebetanz D., Lang N., Antal A., Tergau F., Paulus W. (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology, 114, 2220–2. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Paulus W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527(Pt 3), 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Owen A.M. (2000). The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Experimental Brain Research, 133, 33–43. [DOI] [PubMed] [Google Scholar]
- Pallanti S., Di Rollo A., Antonini S., Cauli G., Hollander E., Quercioli L. (2012). Low-frequency rTMS over right dorsolateral prefrontal cortex in the treatment of resistant depression: cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology, 65, 227–35. [DOI] [PubMed] [Google Scholar]
- Peterson R.A., Heilbronner R.L. (1987). The anxiety sensitivity index: Construct validity and factor analytic structure. Journal of Anxiety Disorders, 1, 117–21. [Google Scholar]
- Quartz S.R. (2009). Reason, emotion and decision-making: Risk and reward computation with feeling. Trends in Cognitive Sciences, 13, 209–15. [DOI] [PubMed] [Google Scholar]
- Reiss S., Peterson R.A., Gursky D.M., McNally R.J. (1986). Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behavior Research and Therapy, 24, 1–8. [DOI] [PubMed] [Google Scholar]
- Resstel L.B., Souza R.F., Guimaraes F.S. (2008). Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiology and Behavior, 93, 200–5. [DOI] [PubMed] [Google Scholar]
- Rodriguez B.F., Bruce S.E., Pagano M.E. (2004). Factor structure and stability of the Anxiety Sensitivity Index in a longitudinal study of anxiety disorder patients. Behaviour Research and Therapy, 42, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12, 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35, 169–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa P., Leiva A.P.G., Castro C.D.C., et al. (2014). Transcranial direct current stimulation for generalized anxiety disorder: a case study. Biological Psychiatry, 75, e17–8. [DOI] [PubMed] [Google Scholar]
- Simon J.J., Walther S., Fiebach C.J., et al. (2010). Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage, 49, 1868–74. [DOI] [PubMed] [Google Scholar]
- Spielberg J.M., Miller G.A., Warren S.L., et al. (2012). A brain network instantiating approach and avoidance motivation. Psychophysiology, 49, 1200–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Nitsche M.A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17, 37–53. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Paulus M.P. (2009). Imbalance of approach and avoidance: The yin and yang of anxiety disorders. Biological Psychiatry, 66, 1072–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S.K., Davidson R.J. (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8, 204–10. [Google Scholar]
- Tadini L., El-Nazer R., Brunoni A.R., et al. (2011). Cognitive, mood, and electroencephalographic effects of noninvasive cortical stimulation with weak electrical currents. Journal of ECT, 27, 134–40. [DOI] [PubMed] [Google Scholar]
- Talmi D., Dayan P., Kiebel S.J., Frith C.D., Dolan R.J. (2009). How humans integrate the prospects of pain and reward during choice. Journal of Neuroscience, 29, 14617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato C., Piccione F., Cavinato M., et al. (2013). Modulation of affective symptoms and resting state activity by brain stimulation in a treatment resistant case of obsessive-compulsive disorder. Eurocase, 19, 360–70. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–70. [DOI] [PubMed] [Google Scholar]