Abstract
Background
After more than a decade of steadily declining notifications, the number of reported cholera cases has recently increased in Vietnam. We conducted a matched case-control study to investigate transmission of cholera during an outbreak in Ben Tre, southern Vietnam, and to explore the associated risk factors.
Methodology/Principal findings
Sixty of 71 diarrheal patients confirmed to be infected with cholera by culture and diagnosed between May 9 and August 3, 2010 in Ben Tre were consecutively recruited as case-patients. Case-patients were matched 1:4 to controls by commune, sex, and 5-year age group. Risk factors for cholera were examined by multivariable conditional logistic regression. In addition, environmental samples from villages containing case-patients were taken to identify contamination of food and water sources. The regression indicated that drinking iced tea (adjusted odds ratio (aOR) = 8.40, 95% confidence interval (CI): 1.84–39.25), not always boiling drinking water (aOR = 2.62, 95% CI: 1.03–6.67), having the main source of water for use being close to a toilet (aOR = 4.36, 95% CI: 1.37–13.88), living with people who had acute diarrhea (aOR = 13.72, 95% CI: 2.77–67.97), and little or no education (aOR = 4.89, 95% CI: 1.18–20.19) were significantly associated with increased risk of cholera. In contrast, drinking stored rainwater (aOR = 0.17, 95% CI: 0.04–0.63), eating cooked seafood (aOR = 0.27, 95% CI: 0.10–0.73), and eating steamed vegetables (aOR = 0.22, 95% CI: 0.07–0.70) were protective against cholera. Vibrio cholerae O1 Ogawa carrying ctxA was found in two of twenty-five river water samples and one of six wastewater samples.
Conclusions/Significance
The magnitude of the cholera outbreak in Ben Tre was lower than in other similar settings. This investigation identified several risk factors and underscored the importance of continued responses targeting cholera prevention in southern Vietnam. The association between drinking iced tea and cholera and the spread of V. cholerae O1, altered El Tor strains warrant further research. These findings might be affected by a number of limitations due to the inability to capture asymptomatic or mildly symptomatic infections, the possible underreporting of personal unhygienic behaviors, and the purposive selection of environmental samples.
Author summary
Cholera is a highly contagious, acute diarrheal illness, which poses a profound health threat in many parts of the less developed world. The majority of cases are reported from Sub-Saharan Africa, South-East Asia, and the Americas (i.e., Haiti) where infections are primarily transmitted through ingestion of contaminated water. Today in the era of widely available rehydration therapies and antibiotics, deaths due to cholera are quite rare. Despite this, early detection of contaminated water sources is crucial for directing early interventions for curbing community-wide transmission. The authors found evidence linking an outbreak of cholera in southern Vietnam to consumption of unsafe water, especially drinking iced tea. This finding suggests the need for a water-monitoring system at ice-making plants. Further research is needed to confirm the biological link between iced tea consumption and cholera infection. Larger studies should also be conducted to understand the clinical consequences of infection with the new cholera agent (V. cholerae O1, altered El Tor strains).
Introduction
Cholera is a highly contagious diarrheal disease, caused by infection of the Gram-negative bacterium Vibrio cholerae [1]. Areas with poverty, high population-density, poor sanitation, poor education levels, and lack of potable water are at risk for cholera outbreaks [2–4]. An estimated 1.3–4.0 million illnesses and 21,000–143,000 deaths are attributed directly to the disease, which is predominantly seen in Sub-Saharan Africa, South-East Asia, and the Americas (i.e., Haiti) [5]. Consumption of contaminated water is thought to be the main mode of transmission [6–8].
Prior to implementation of control measures, Vietnam suffered a disproportionate burden of cholera. For example, between 1979 and 1996, there were 56,050 reported cases of cholera and 1,272 deaths due to cholera in this country [9]. Oral cholera vaccination programs were introduced in highly endemic areas through a national expanded program of immunization in 1997 [10]. The program, in conjunction with improved personal hygiene and access to potable drinking water associated with both health promotion programs and economic growth, led to a substantial drop in the number of notified cholera cases [6, 10]. However, these gains have not been sustained. Resource constraints [10] as well as the modest vaccine effectiveness, estimated to be 76% during the outbreak and 50% after 3–5 years [11, 12], have jeopardized the long-term impact of this program.
There were a total of 3,646 cholera cases reported between 2006 and 2010 in Vietnam, which was nearly five times higher than the number of notification made during 2001–2005 (747 cases) [13]. Of concern, from both a clinical and public health perspective, is the emergence and transmission of V. cholerae O1, El Tor strains that produce the classical cholera toxin [14] and the circulation of strains genetically resistant to certain antibiotics [9, 15]. Outbreaks in Hanoi and neighboring regions in northern Vietnam in 2007–2008 have been linked to contaminated food, especially dog meat, raw vegetables, and raw pig/duck blood [11, 13]. However, the pathways for the transmission of cholera in other parts of the country have not yet been investigated.
In this article, we report a cholera outbreak in the Mekong Delta province of Ben Tre in 2010. We conducted a matched case-control study to identify risk factors for cholera infection, and examined environmental samples to identify contaminated food and water sources. These data are useful for informing targeted and effective responses to cholera outbreaks in Vietnam.
Methods
Study settings
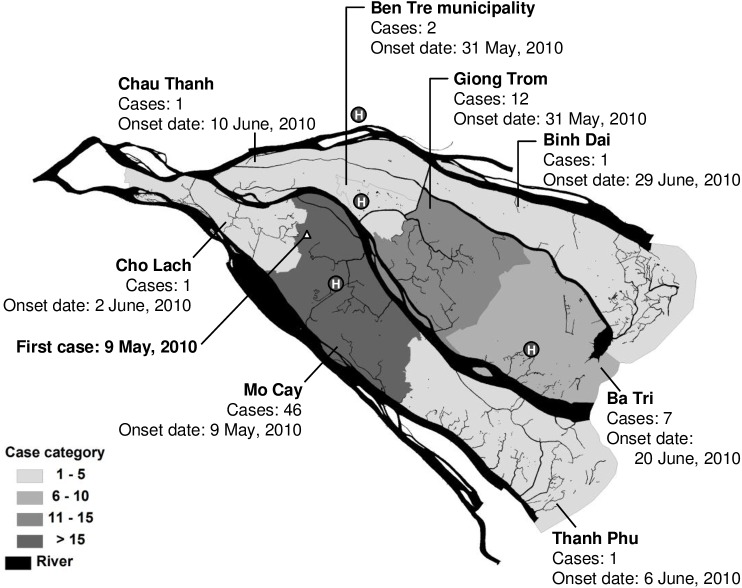
Ben Tre province is located in the Mekong Delta region of southern Vietnam. It has a population of 1.3 million people and a largely agricultural economy. This coastal province is about 1.25 meters above sea level and is almost surrounded by water (Fig 1). Due to low piped-water coverage, river water is generally used as one of the main sources of water among the population. The last mass oral cholera vaccine program was implemented in 2002, and no cholera cases had been reported since 2005 [10, 16]. However, in 2010, an outbreak of cholera occurred in the province, starting in Mo Cay town on May 9. A total of 71 cases were identified in the outbreak (Fig 1).
Fig 1. Map showing the location of 71 culture confirmed cholera cases in Ben Tre, Vietnam during May-August 2010.
The triangle represents the first case of cholera identified in May 9, 2010 in Tan Phu Tay commune, northern Mo Cay district, Ben Tre province. Letter H inside a black circle represents an assigned cholera treatment center in this outbreak.
Collection of food and water samples
We employed a purposive sampling technique to collect water samples from several types of water sources in Mo Cay and Giong Trom towns, where the first cholera cases were reported in this outbreak. They included 25 samples of river water obtained from the rivers closest to case-patients’ houses; six samples of indoor water, six wastewater samples, and two samples of drinking water in case-patients’ houses. In addition, 27 samples of fresh seafood were obtained from local markets where the case-patients lived.
Case-control study
From June 4 through July 2, 2010, we conducted a matched case-control study with a target sample size of 60 case-patients and 240 controls. Because the outbreak occurred in a not well-defined population and required rapid investigation, the case-control study would be the most appropriate choice of study design to identify risk factors for cholera infection [17]. In this study, the matching by age groups, sex, and living areas was used [18]. The sample size of this study for 80% power at 5% level of significance was calculated on the basis of the estimated percentage of controls exposed to unsafe water (30%), the ratio of controls to cases (4), and an odds ratio (OR, 2.25) as per the method of Kelsey and colleagues [19]. The estimated magnitude of OR that we used to calculate the sample size came from the previous work of Hoge and his colleagues [8]. The smallest reported OR was selected to ensure that the study was large enough and had sufficient statistical power in identifying risk factors with an expected OR of 2.25 or higher.
During this outbreak, all patients with acute watery diarrhea who sought diagnosis and treatment for their disease at local health facilities were immediately transferred to the nearest cholera treatment center. In all, there were four centers, which were established soon after the outbreak was confirmed and located in three distant local hospitals and one neighboring hospital (Fig 1). In these centers, case-patients were identified, quarantined, and treated, and they were considered eligible for inclusion if they had laboratory-confirmed V. cholerae identified through conditional culture of rectal swabs and resided permanently in Ben Tre province. For this study, case-patients identified since the beginning of the outbreak were consecutively recruited until the target sample size was reached. Case-patients who were under cholera treatment were prospectively recruited through the four cholera treatment centers, while case-patients who had already been discharged from these health facilities were contacted and recruited at their homes.
For each case, four community-based controls [20] were selected and matched by commune, sex and 5-year age group to control for the potential confounding effects of these three factors. They were recruited at their homes on the same day as the interview of their matched case. Specifically, the control search began with random selection of four sub-communes in the area where the case resided by using Microsoft Excel. In each of these sub-communes, currently registered houses were numbered and one house was then chosen by hand drawing from the Vietnamese bingo game, a set containing 90 balls numbered 1–90. Interviewers subsequently accessed the selected house, in which all household members were primarily screened by sex and age to identify a potential control. Controls were further screened by interviewers to ensure that they had not had acute diarrhea in the month prior to the interview. If no person in that house met the inclusion criteria, the interviewers went to the next house to the left until a matched control was found. No control persons that we approached refused to participate in the present study and be interviewed.
Trained health-workers used a structured questionnaire to collect the study information during face-to-face interviews. This questionnaire was adapted from a cholera case investigation form previously developed and used in Ho Chi Minh City in 2008. For the present study, it was extended and included a section for controls. Risk variables included were based on reports in the literature [8, 21] and primary assessments conducted at the beginning of the outbreak. The questionnaire contained questions to elicit information on socio-demographic characteristics, exposure to people with diarrheal diseases, recent travel history, and detailed consumption of food and water. The logic and language of questions were tested locally in Ben Tre before being used to collect data. For children aged <6 years, interviews were conducted with their parents or guardians. Potential contaminated food sources were identified through dichotomous questions about the different types of food eaten, eating places, and cooking methods (e.g., well-cooked, stir fried, cooked rare, and raw) within five days before the onset of diarrhea for case-patients or five days before being interviewed for control persons. Participants were also asked about their sources and characteristics of water used for drinking, cooking, and bathing, and their drinking habits (e.g., the use of boiled water and drinking water with ice) via several dichotomous questions in the previous seven days. Clinical symptoms and health-seeking behaviors since the onset of diarrheal symptoms were also collected. Rectal swabs were collected from controls after completion of the interview.
Laboratory analysis
During the outbreak, rectal swabs of people with acute diarrhea were directly placed in Cary Blair Transport Medium and transported daily to the Pasteur Institute, Ho Chi Minh City (PI-HCMC) for identification of V. cholerae using a standard testing protocol [22, 23]. All controls’ rectal swabs and environmental samples were stored at the Ben Tre Provincial Preventive Centre and transported fortnightly to the PI-HCMC for testing. Specifically, swab specimens were enriched in alkaline peptone water (Oxoid) and inoculated for 6–8 hours. We then cultured the bacterial solution obtained on thiosulfate citrate bile salts sucrose agar (Merck) and carried out biochemical and agglutination tests (Denka Seiken). We further used polymerase chain reaction (PCR) to amplify the V. cholera isolations from the swab culture, environmental samples, to identify the genomic region encoding the O1/O139 genotypes, and to detect cholera toxins and other toxin-specific genes, specifically ctxA, ctxB, rtxC, and rstC, using previously published primers [24–28] according to the PCR method described by Nguyen et al.[29]. A 9 μl of amplicon was separated by electrophoresis on a 1.5% agarose gel and visualized with an ultraviolet light on the Gel Doc System (Bio-Rad Laboratories). Test results were immediately reported to the attending physicians and the local preventive centers for personal treatment and prevention purposes, respectively.
Ethical statements
In Vietnam, the emergence of cholera is a significant health threat that requires a rapid public health response to protect the entire community from this severe, highly contagious diarrheal disease, and minimize any negative domino effects on population health. For this reason and in view of case-patients’ consent for cholera diagnosis and treatment at hospitals and the minimal risks to the subjects in this outbreak investigation, our request of a waiver of documentation of written informed consent for all participants was reviewed and approved by the local health authority in Ben Tre. A prepared sample verbal consent script was read to the participants. Verbal consents were obtained from all participants and documented through the use of a record of names kept in a secure and locked cabinet at the PI-HCMC. The outbreak investigation and study procedures have been reviewed and ratified by the PI-HCMC Institutional Review Board (reference number: 16/CN-HĐĐĐ).
Statistical analysis
Statistical analysis was carried out using Stata version 14 (StataCrop LP, College Station, TX). Information about the participants in the case-control study were initially explored using descriptive statistics (including frequency and proportion for categorical variables and median and range for continuous variables), with comparisons between case-patients and controls made by McNemar’s chi-square tests. Children aged <6 years were classified as not having consumed a certain food or water if their parents or guardians reported that they did not consume it over the study period. To identify risk factors for cholera, conditional logistic regression with forward selection was used to estimate matched OR and 95% confidence intervals (CI) as per the method of Hosmer and his colleagues [30]. Initially, univariate analyses were employed to identify variables that were potential risk factors for cholera infection. Those variables with p<0.25, along with those which have been acknowledged to have biologic plausibility for increasing the risk of cholera, were considered for inclusion in the multivariable model. The analysis began by fitting the base model, which included cholera variable, participants’ age, and a variable that yielded the lowest corresponding p-value in univariate analyses. The variable with the next lowest p-value was progressively added to the model, and its contribution to model fit was tested by the log likelihood ratio test. If the test yielded a p-value less than 0.05, it was kept in the model. This process continued until the added variable made no significant improvement in model fit. As the level of education may behaviorally influence personal eating and drinking habits, we investigated potential effect modification by creating interaction terms between it and the other variables retained in the final model. In a sensitivity analysis, to accommodate missing data, we applied multiple imputation by chained equations, with an assumption of missingness at random. Ten imputed datasets were used with 1,000 iterations. We compared the risk factors found between the original and imputed data sets.
Results
The outbreak
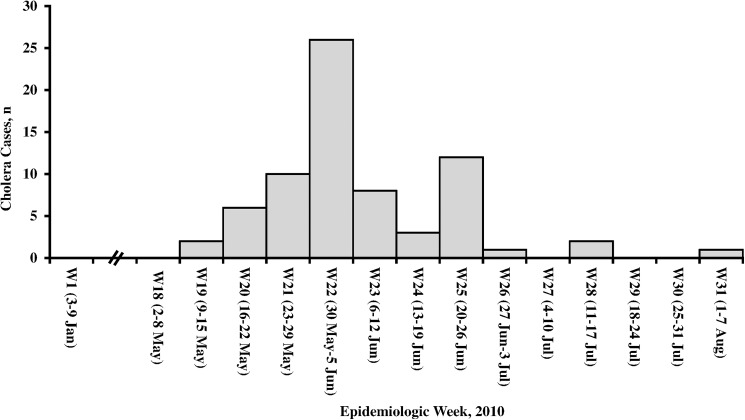
From May 9 (epidemiologic week 19) through August 3 (epidemiologic week 31), 2010, 71 cases of cholera were identified in all towns of Ben Tre province. The area with the highest number of cases was Mo Cay (46 cases), followed by Giong Trom (12 cases) and Ba Tri (7 cases) (Fig 1). The number of cases continued to increase, peaked during May 30 to June 5 (epidemiologic week 22), and then gradually decreased over time (Fig 2). No case-patient died during the outbreak.
Fig 2. Distribution of culture confirmed cholera cases in Ben Tre, Vietnam, by epidemiologic week in 2010.
The start and end dates of each epidemiologic week are shown.
After confirmation of the outbreak on May 12, 2010, Ben Tre rapidly rolled-out its control activities in the entire province. It included measures to isolate and treat case-patients at four cholera treatment centers, clean and disinfect their houses with chloramine B, and provide chloramine B to enhance the use of chloramine-treated water among people living in the areas where the case-patients emerged. Case-patient’s close contacts were traced, given a single dose of prophylactic ciprofloxacin, and monitored their health. Education campaigns were additionally made to encourage people in the entire province to practice safe water, proper sanitation, and food safety. As reports of primary risk assessments suggested a potential link between drinking iced water and the illness, on June 11, 2010, the government of Ben Tre prohibited the manufacture, transportation, sale, or supply of commercial ice in the two most affected towns of Mo Cay and Giong Trom.
Laboratory and environmental results
In total, only V. cholerae O1 Ogawa, El Tor biotype was isolated from 71cholera cases. The ctxA, ctxB, rtxC, and rstC genes were exhibited in 97%, 100%, 100%, and 97% of all isolates, suggesting a presence of altered El Tor strains that combine characteristics of classical and El Tor strains. Due to resource constraints, only 193 rectal swabs taken from 240 controls were tested for cholera. V. cholerae was not detected in any controls’ rectal swabs.
Two out of twenty five river water samples and one out of six wastewater samples were culture positive for V. cholerae. PCR analysis revealed the presence of V. cholerae O1 Ogawa carrying ctxA in all of these three positive-culture samples. V. cholerae was absent in two samples of drinking water, six samples of indoor water, and twenty-seven samples of fresh seafood.
Case-control investigation
After excluding seven case-patients lost to follow-up and four case-patients whose disease occurred after the target sample size had been reached, a total of 60 case-patients occurring from May 9 through June 26 were consecutively included in a matched case-control study and matched to 240 controls. Forty case-patients were prospectively recruited through the cholera treatment centers and twenty case-patients were recruited at their homes. The case-patients were interviewed from June 4 through July 2, 2010. Nine case-patients and 32 control persons had missing data on at least one variable. Except for age, no significant discrepancies were recorded between the missing and non-missing subjects on variables (Table A in S1 File).
Demographic features and risk factors are listed in Table 1. Two thirds of subjects were females. Participants’ ages ranged from two months to 83 years for the case-patients and from one month to 87 years for the controls. For those aged six years or older, 73% of the case-patients and 57% of the controls reported having little education (primary education or illiterate).
Table 1. Characteristics of the matched case-control population.
| Variablea | Case patients (N = 60) Frequency (%) |
Controls (N = 240) Frequency (%) |
pb |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age (years), median (range) | 45.5 (0.2–83) | 42 (0.1–87) | - |
| Sex | - | ||
| Female | 40 (67) | 160 (67) | |
| Male | 20 (33) | 80 (33) | |
| Education levelc | <0.001 | ||
| Secondary or higher | 15 (27) | 94 (43) | |
| Primary education or illiterate | 40 (73) | 125 (57) | |
| Hygiene | |||
| Facility for human fecal waste disposal | |||
| Fishpond or river toilets | 44 (73) | 174 (73) | 0.825 |
| Outside latrine | 2 (3) | 3 (1) | 0.227 |
| Flush toilet | 13 (22) | 60 (25) | 0.404 |
| Main source of water close to a toilet | 18 (30) | 33 (14) | <0.001 |
| Self-perceived changes in the color, odor, appearance and taste of water | 9 (15) | 20 (8) | 0.029 |
| Bathes with sedimented river water | 31 (52) | 71 (30) | <0.001 |
| Brushes teeth/gargles with sedimented river waterc | 20 (33) | 38 (16) | <0.001 |
| Drinking water | |||
| Drinks iced tea | 13 (22) | 6 (3) | <0.001 |
| Drinks water with icec | 0.001 | ||
| Never | 11 (19) | 75 (32) | |
| Sometimes, often or always | 48 (81) | 159 (68) | |
| Drinks boiled waterc | <0.001 | ||
| Always | 21 (35) | 136 (57) | |
| Sometimes, often or never | 39 (65) | 102 (43) | |
| Drinks sedimented river water | 7 (12) | 12 (5) | 0.014 |
| Drinks stored rainwaterc | 42 (70) | 212 (89) | <0.001 |
| Drinks bottled water | 12 (20) | 31 (13) | 0.027 |
| Drinks indoor tap water | 2 (3) | 9 (4) | 1.000 |
| Food exposure/practices | |||
| Uses sedimented river water for cooking | 25 (42) | 51 (21) | <0.001 |
| Uses stored rainwater for cooking | 14 (23) | 98 (41) | <0.001 |
| Uses bottled water for cooking | 0 (0) | 2 (1) | 0.500 |
| Uses indoor tap water for cooking | 4 (7) | 28 (12) | 0.058 |
| Eats cooked seafood | 37 (62) | 194 (81) | <0.001 |
| Eats raw seafood or seafood cooked rare | 3 (5) | 8 (3) | 0.503 |
| Eats steamed vegetables | 7 (12) | 74 (31) | <0.001 |
| Eats raw vegetables | 14 (23) | 76 (32) | 0.027 |
| Eats fruits | 7 (12) | 17 (7) | 0.090 |
| Others | |||
| Lives with people who had acute diarrhea | 9 (15) | 5 (2) | <0.001 |
| Travels out of townc | 21 (37) | 48 (20) | <0.001 |
aFood consumption was collected for the 5 days before the onset of diarrhea for case-patients or before being interviewed for controls. Frequent water exposures and other risk behaviors and practices were collected in the previous seven days.
bP-values obtained from McNemar’s chi-square tests.
cDenominators may be lower than the total number because of missing data.
The median number of case-patient’s stools within a day prior to hospital admission was eight (range: 2–20), 55% had watery stools, 40% had abdominal pain, and 53% had vomiting. The mean time of admission to local hospitals was 0.7 days after the onset of the disease, and the mean time lag between case onset and enrolment of controls was 12 days among the case-patients.
Fishpond or river toilets were frequent in both the case-patient’s and control’s houses (73% and 73%, respectively). A flush toilet in house was reported in 22% of the case-patients and 25% of the controls (p = 0.404). Thirty percent of the case-patients reported that their main sources of water for use were close to a toilet, whereas only 14% of the controls had a similar situation (p <0.001). A self-report of changes in the color, odor, appearance and taste of water used was seen in 15% of the case-patients and 8% of the controls (p = 0.029).
Among the case-patients surveyed, 22% reported drinking iced tea within one week before the disease onset, compared with 3% of the controls in the week before being interviewed (p <0.001). A higher percentage of the case-patients than the controls consumed water with ice (81% vs. 68%, p = 0.001), unboiled water (65% vs. 43%, p <0.001), and sedimented river water (12% vs. 5%, p = 0.014). Up to 52%, 33%, and 42% of the case-patients used sedimented river water as the source of water for bathing, brushing teeth/gargling, and cooking, respectively. These levels were nearly twice as high as those for the controls (30%, 16%, and 21%, respectively; all p-values <0.001). The case-patients were less likely to use stored rainwater for both drinking (70% vs. 89%, p <0.001) and cooking (23% vs. 41%, p <0.001), but they were more likely to drink bottled water (20% vs. 13%, p = 0.027) than the controls. The use of indoor tap water for both drinking and cooking was rarely seen in this sample.
The case-patients were less likely to eat cooked seafood (62% vs. 81%, p<0.001), steamed vegetables (12% vs. 31%, p <0.001) and raw vegetables (23% vs. 32%, p = 0.027) in comparison with the controls.
While only 2% of the controls were living with people who had acute diarrhea, 15% of the case-patients did so (p <0.001). The case-patients had a greater percentage of travel out of town in the week prior to the disease onset than the controls (37% vs. 20%, p <0.001).
Risk factors for cholera infection identified through the multivariable conditional logistic regression are summarized in Table 2. Individuals who reported drinking iced tea (adjusted OR (aOR) = 8.40, 95% CI: 1.84–39.25), not always boiling drinking water (aOR = 2.62, 95% CI: 1.03–6.67), living with people who had acute diarrhea (aOR = 13.72, 95% CI: 2.77–67.97), having the main household sources of water for use close to a toilet (aOR = 4.36, 95% CI: 1.37–13.88), and having little or no education (aOR = 4.89, 95% CI: 1.18–20.19) were significantly associated with an elevated risk of cholera. The risk of cholera was lower among persons who drank stored rainwater (aOR = 0.17, 95% CI: 0.04–0.63), ate cooked seafood (aOR = 0.27, 95% CI: 0.10–0.73), and ate steamed vegetables (aOR = 0.22, 95% CI: 0.07–0.70). No significant interactions were recorded throughout the analysis. Only the level of education become insignificant in another regression analysis based on a multiple imputation approach (Table B in S1 File).
Table 2. Conditional logistic regression analysis of cholera risk factors in Ben Tre, southern Vietnam, 2010.
| Variable | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|
| Crude OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Sociodemographic factors | ||||
| Age (years)b | 1.07 (0.99–1.15) | 0.074 | 1.07 (0.97–1.17) | 0.187 |
| Education level | ||||
| Secondary or higher | ref | ref | ||
| Primary education or illiterate | 3.49 (1.32–9.22) | 0.012 | 4.89 (1.18–20.19) | 0.028 |
| Hygiene | ||||
| Flush toilet in house | 0.82 (0.40–1.66) | 0.574 | n/ac | n/ac |
| Main source of water close to a toilet | 3.60 (1.62–7.99) | 0.002 | 4.36 (1.37–13.88) | 0.013 |
| Self-perceived changes in the color, odor, appearance and taste of water | 1.99 (0.84–4.71) | 0.119 | - | - |
| Bathes with sedimented river water | 2.84 (1.52–5.31) | 0.001 | 2.33 (0.93–5.82) | 0.070 |
| Brushes teeth/gargles with sedimented river water | 2.80 (1.44–5.48) | 0.003 | - | - |
| Drinking water | ||||
| Drinks iced tea | 11.89 (3.85–36.70) | <0.001 | 8.40 (1.84–39.25) | 0.006 |
| Drinks water with ice | ||||
| Never | ref | |||
| Sometimes, often or always | 2.11 (1.01–4.40) | 0.046 | - | - |
| Drinks boiled water | ||||
| Always | ref | ref | ||
| Sometimes, often or never | 2.71 (1.44–5.09) | 0.002 | 2.62 (1.03–6.67) | 0.044 |
| Drinks sedimented river water | 2.58 (0.94–7.06) | 0.064 | - | - |
| Drinks stored rainwater | 0.25 (0.12–0.53) | <0.001 | 0.17 (0.04–0.63) | 0.008 |
| Drinks bottled water | 1.78 (0.81–3.90) | 0.149 | - | - |
| Drinks indoor tap water | 0.89 (0.19–4.11) | 0.880 | n/ac | n/ac |
| Food exposure/practices | ||||
| Uses sedimented river water for cooking | 2.93 (1.54–5.58) | 0.001 | - | - |
| Uses stored rainwater for cooking | 0.39 (0.20–0.79) | 0.008 | - | - |
| Uses indoor tap water for cooking | 0.40 (0.11–1.54) | 0.184 | - | - |
| Eats cooked seafood | 0.37 (0.20–0.70) | 0.002 | 0.27 (0.10–0.73) | 0.009 |
| Eats raw seafood or seafood cooked rare | 1.50 (0.40–5.65) | 0.549 | n/ac | n/ac |
| Eats steamed vegetables | 0.26 (0.11–0.61) | 0.002 | 0.22 (0.07–0.70) | 0.011 |
| Eats raw vegetables | 0.64 (0.32–1.26) | 0.195 | - | - |
| Eats fruits | 1.97 (0.69–5.61) | 0.207 | - | - |
| Others | ||||
| Lives with people who had acute diarrhea | 7.20 (2.41–21.48) | <0.001 | 13.72 (2.77–67.97) | 0.001 |
| Travels out of town | 2.57 (1.26–5.23) | 0.009 | - | - |
Reference is the absence of a characteristic, unless otherwise specified. CI, confidence interval; OR, odds ratio.
aThe analysis was based on 265 observations with complete data. The estimated pseudo-R-squared of the final model was 44.9%.
bAge was modeled as a continuous variable.
cThis variable was not selected for multivariate analysis due to a corresponding p-value above 0.25 in univariate analysis.
Discussion
In this investigation of the 2010 Ben Tre cholera outbreak, most risk factors identified have been previously described and associated with exposure to unsafe water (such as, not always drinking boiled water, main source of water close to toilet) or with proximity with another possible case who had acute diarrhea. Our results revealed a significantly increased risk of cholera among individuals who reported drinking iced tea in the week before the onset of their illness. We also observed substantial cholera risk reductions in some subpopulations, specifically those who drank stored rainwater and ate cooked seafood or steamed vegetables.
Our study suggested that the spread of cholera in Ben Tre was quite considerable, but not nearly as high as had been seen in Hanoi [11] and other Southeast Asian settings [31, 32]. Early outbreak detection, community-engaged health promotion, large-scale distribution of chloramine B to treat surface water, its low population density (an estimated 533 people/km2), and a prohibition of ice block production business during the outbreak could have contributed to the relatively smaller size of the outbreak in Ben Tre province. In agreement with previous studies [14, 26, 32, 33], we found evidence that V. cholerae O1, altered El Tor was prevalent among the case-patients. There were no reported deaths attributable to any strains of cholera among the case-patients in this outbreak. This finding could help to address the previously reported clinical concern that the spread of these strains with increased severity would lead to more cholera deaths in some areas of the world [34].
We know of no previous studies that have reported increased risk of infection specifically associated with iced tea consumption. Locally, iced tea is made by adding cooled boiled tea to a glass or bottle of ice. Ice is typically bought from street vendors rather than prepared at home in rural or semi-rural areas of Vietnam because less than one-eighth (12%) of rural households own a refrigerator [35]. A previous hospital-based case-control study conducted in Thailand demonstrated that consumption of ice was significantly associated with increased risk of cholera in a bivariate analysis but was not significant in the multivariable model [8]. In our outbreak investigation, we were not able to obtain commercial and household ice for testing for the presence of V. cholerae in ice. We were therefore unable to ascertain the underlying microbiological mechanism leading to the association between drinking iced tea and cholera. A previous study, conducted in Jakarta, Indonesia, showed that a large percentage of ice (35%) and beverages (24%) were contaminated with V. cholerae [36]. In Vietnam where untreated wells and surface water (i.e. river water) have been commonly used for making commercial ice, it is possible for V. cholerae to be introduced into commercial ice, and such contamination could have triggered this resurgence of cholera in Ben Tre province. The observation that the decline in the weekly reported number of cholera was seen after the local government prohibited on the manufacture, transportation, sale, or supply of commercial ice in the outbreak area supports this hypothesis. In future outbreaks, further epidemiological and microbiological investigation of the association between commercial ice and cholera is warranted. The microbiological quality of the water used at ice manufacturing plants should be tested regularly.
Our other risk factors for cholera included living with people who had acute diarrheal illness and not always boiling drinking water. These are well-described predictors of cholera in endemic countries [8, 37]. The association between having the main source of water for use being close to the toilets indicates that pollution of drinking water sources by contaminated feces remains a problem in Vietnam and could lead to further spread of disease in future cholera outbreaks [38]. As expected, we found that drinking stored rainwater, eating cooked seafood, and eating steamed vegetables were protective against cholera, and these results are consistent with findings from several previous studies [21, 39]. Local public health authorities should rapidly roll out a response that involves appropriate case management and greater promotion of proper sanitation, safe water, and food safety in the event of a cholera outbreak [40].
Our observation that individuals who reported little or no education were at a higher risk of cholera is consistent with the results of a previous case-control study in Harare City, Zimbabwe in which having attained less than secondary education was found to be a risk factor for cholera [41]. Poor education is part of the cycle of poverty, which includes crowded living conditions, malnourishment, poor household sanitation and personal hygiene practices [42], which are well-known to be important risk factors for cholera [43].
This study has several limitations. First, the number of cholera cases reported herein might not have reflected the actual burden of cholera in Ben Tre province. The vast majority of people infected with V. cholerae are asymptomatic or mildly symptomatic, and thus might not seek healthcare services [44]. Second, detection of V. cholerae in some water samples obtained only from Mo Cay and Giong Trom should be interpreted with caution, because the degree to which these samples are representative of environmental sources in other towns throughout the province where case-patients occurred is unknown. Third, selection of controls from the commune where the case-patients resided can limit exploration of some important geographical and cultural risk factors, such as exposure to river water, due to similar exposures between the controls and the case-patients. Fourth, reduced recall of information may have occurred among case-patients recruited and interviewed late. To reduce the need to recall distant exposures among both the case-patients and controls, study questions were specific to food/water and location. Fifth, about 20% of controls’ rectal swabs were not tested for cholera, potentially misclassifying case-patients as controls. However, such misclassification was unlikely as 80% of controls’ rectal swabs tested were negative for V. cholerae. Sixth, as with other behavioral studies that depend entirely on face-to-face interviews, this study may under-report some important risks, such as food and personal hygiene practices. To reduce this bias, we selected well-trained, experienced, and unprejudiced interviewers after the interview training. Lastly, our study may not have enough power to detect rare risk factors for cholera.
Despite these limitations, this present study has important implications for Vietnam’s cholera responses. This emergence of cholera due to V. cholerae O1, altered El Tor emphasizes the need for increased efforts to prevent the spread of cholera in southern Vietnam. Along with traditional approaches that focus on enhancement of safe water, sanitation, and food safety, combined with periodic provision of oral cholera vaccines, a water quality monitoring system at ice-making plants should be established. It is vital to ensure the quality of the water supply, reduce the introduction of V. cholerae into ice, and subsequently lower the risk of cholera in Vietnam, a tropical setting where consumption of iced drinks is common.
Supporting information
(PDF)
Characteristics of the sample with and without missing data (Table A). Cholera risk factors in Ben Tre, southern Vietnam, 2010 (Table B).
(DOCX)
Acknowledgments
The authors wish to thank Dr. Nguyen Thi Phuong Lan (Department of Microbiology and Immunology), Dr. Nguyen Thi Minh Phuong, Dr. Nguyen Quoc Huy, Dr. Vo Ngoc Quang, and Mr. Doan Ngoc Minh Quan (Department for Disease Control and Prevention), and other health professionals at the Pasteur Institute, Ho Chi Minh City involved in this investigation; and Dr. Ho Trung Tuyen and other health professionals at the Provincial and District-level Preventive Medicine Centers in Ben Tre for their contributions and support. The authors also wish to thank Dr. Andrew P. Craig at the University of Cambridge and Dr. Matthew R. Moore at the U.S. Centers for Disease Control and Prevention for helpful discussion and assistance with English language editing and proof-reading before submission. The findings and conclusions in this report do not necessarily represent the position of the authors’ institutions, of the Pasteur Institute, Ho Chi Minh City, and of the Vietnamese Ministry of Health.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was originally supported by the Vietnam's Ministry of Health. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nelson EJ, Harris JB, Morris JG Jr., Calderwood SB, Camilli A. Cholera transmission: The host, pathogen and bacteriophage dynamic. Nat Rev Microbiol 2009,7:693–702. doi: 10.1038/nrmicro2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, Morris JG, Levine MM. Cholera. Clin Microbiol Rev 1995,8:48–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackers M-L, Quick RE, Drasbek CJ, Hutwagner L, Tauxe RV. Are there national risk factors for epidemic cholera? The correlation between socioeconomic and demographic indices and cholera incidence in Latin America. Int J Epidemiol 1998,27:330–334. [DOI] [PubMed] [Google Scholar]
- 4.Ali M, Emch M, Donnay JP, Yunus M, Sack RB. Identifying environmental risk factors for endemic cholera: A raster GIS approach. Health Place 2002,8:201–210. [DOI] [PubMed] [Google Scholar]
- 5.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 2015,9:e0003832 doi: 10.1371/journal.pntd.0003832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly-Hope LA, Alonso WJ, Thiem VD, Canh do G, Anh DD, Lee H, et al. Temporal trends and climatic factors associated with bacterial enteric diseases in Vietnam, 1991–2001. Environ Health Perspect 2008,116:7–12. doi: 10.1289/ehp.9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen VD, Sreenivasan N, Lam E, Ayers T, Kargbo D, Dafae F, et al. Cholera epidemic associated with consumption of unsafe drinking water and street-vended water—Eastern Freetown, Sierra Leone, 2012. Am J Trop Med Hyg 2014,90:518–523. doi: 10.4269/ajtmh.13-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoge CW, Bodhidatta L, Echeverria P, Deesuwan M, Kitporka P. Epidemiologic study of Vibrio cholerae O1 and O139 in Thailand: at the advancing edge of the eighth pandemic. Am J Epidemiol 1996,143:263–268. [DOI] [PubMed] [Google Scholar]
- 9.Dalsgaard A, Forslund A, Tam NV, Vinh DX, Cam PD. Cholera in Vietnam: changes in genotypes and emergence of class I integrons containing aminoglycoside resistance gene cassettes in vibrio cholerae O1 strains isolated from 1979 to 1996. J Clin Microbiol 1999,37:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anh DD, Lopez AL, Tran HTM, Cuong NV, Thiem VD, Ali M, et al. Oral cholera vaccine development and use in Vietnam. PLoS Med 2014,11:e1001712 doi: 10.1371/journal.pmed.1001712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anh DD, Lopez AL, Thiem VD, Grahek SL, Duong TN, Park JK, et al. Use of oral cholera vaccines in an outbreak in Vietnam: A case control study. PLoS Negl Trop Dis 2011,5:e1006 doi: 10.1371/journal.pntd.0001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiem VD, Deen JL, von Seidlein L, Canh DG, Anh DD, Park J-K, et al. Long-term effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine 2006,24:4297–4303. doi: 10.1016/j.vaccine.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen Tran Hien. Emerging and re-emerging infectious diseases in Vietnam. Hanoi, Vietnam: November, 2012.
- 14.Nguyen BM, Lee JH, Cuong NT, Choi SY, Hien NT, Anh DD, et al. Cholera outbreaks caused by an altered Vibrio cholerae O1 El Tor biotype strain producing classical cholera toxin B in Vietnam in 2007 to 2008. J Clin Microbiol 2009,47:1568–1571. doi: 10.1128/JCM.02040-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehara M, Nguyen BM, Nguyen DT, Toma C, Higa N, Iwanaga M. Drug susceptibility and its genetic basis in epidemic Vibrio cholerae O1 in Vietnam. Epidemiol Infect 2004,132:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vietnamese Ministry of Health (MOH). The infectious disease Vietnam statistical yearbooks, 2003–2014. Hanoi, Vietnam: MOH; 2004–2015. [Google Scholar]
- 17.U.S. Centers for Disease Control and Prevention (U.S. CDC). Principles of epidemiology in public health practice: An introduction to applied epidemiology and biostatistic. 3rd ed. Atlanta, GA: U.S. CDC; 2012. [Google Scholar]
- 18.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2009. [Google Scholar]
- 19.Kelsey JL, Whittemore AJ, Evans AS, Thompson WD. Methods in obervational epidemiology. 2nd ed. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 20.Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet 2005,365:1429–1433. doi: 10.1016/S0140-6736(05)66379-9 [DOI] [PubMed] [Google Scholar]
- 21.Shapiro RL, Otieno MR, Adcock PM, Phillips-Howard PA, Hawley WA, Kumar L, et al. Transmission of epidemic Vibrio cholerae O1 in rural western Kenya associated with drinking water from Lake Victoria: an environmental reservoir for cholera? Am J Trop Med Hyg 1999,60:271–276. [DOI] [PubMed] [Google Scholar]
- 22.Raychoudhuri A, Mukhopadhyay AK, Ramamurthy T, Nandy RK, Takeda Y, Nair GB. Biotyping of Vibrio cholerae O1: Time to redefine the scheme. Indian J Med Res 2008,128:695–698. [PubMed] [Google Scholar]
- 23.Parsons MB, Cooper KLF, Kubota KA, Puhr N, Simington S, Calimlim PS, et al. PulseNet USA standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio parahaemolyticus. Foodborne Pathog Dis 2007,4:285–292. doi: 10.1089/fpd.2007.0089 [DOI] [PubMed] [Google Scholar]
- 24.Morita M, Ohnishi M, Arakawa E, Bhuiyan NA, Nusrin S, Alam M, et al. Development and validation of a mismatch amplification mutation PCR assay to monitor the dissemination of an emerging variant of Vibrio cholerae O1 biotype El Tor. Microbiol Immunol 2008,52:314–317. doi: 10.1111/j.1348-0421.2008.00041.x [DOI] [PubMed] [Google Scholar]
- 25.Chow KH, Ng TK, Yuen KY, Yam WC. Detection of RTX toxin gene in Vibrio cholerae by PCR. J Clin Microbiol 2001,39:2594–2597. doi: 10.1128/JCM.39.7.2594-2597.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel AK, Jain M, Kumar P, Jiang SC. Molecular characterization of Vibrio cholerae outbreak strains with altered El Tor biotype from southern India. World J Microbiol Biotechnol 2010,26:281–287. doi: 10.1007/s11274-009-0171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields PI, Popovic T, Wachsmuth K, Olsvik O. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol 1992,30:2118–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 2000,406:477–483. doi: 10.1038/35020000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen DT, Ngo TC, Tran HH, Le TH, Nguyen HT, Nguyen BM, et al. Characterization of Vibrio cholerae O139 of an aquatic isolate in northern Vietnam. Open Microbiol J 2012,6:14–21. doi: 10.2174/1874285801206010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. New York, NY: John Wiley & Sons; 2013. [Google Scholar]
- 31.Sithivong N, Morita-Ishihara T, Vongdouangchanh A, Phouthavane T, Chomlasak K, Sisavath L, et al. Molecular subtyping in cholera outbreak, Laos, 2010. Emerg Infect Dis 2011,17:2060–2062. doi: 10.3201/eid1711.110280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwood PF, Collins D, Jonduo MH, Rosewell A, Dutta SR, Dagina R, et al. Clonal origins of Vibrio cholerae O1 El Tor strains, Papua New Guinea, 2009–2011. Emerg Infect Dis 2011,17:2063–2065. doi: 10.3201/eid1711.110782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair GB, Qadri F, Holmgren J, Svennerholm A-M, Safa A, Bhuiyan NA, et al. Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol 2006,44:4211–4213. doi: 10.1128/JCM.01304-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddique AK, Nair GB, Alam m, Sack DA, Huq A, Nizam A, et al. El Tor cholera with severe disease: A new threat to Asia and beyond. Epidemiol Infect 2010,138:347–352. doi: 10.1017/S0950268809990550 [DOI] [PubMed] [Google Scholar]
- 35.General Statistical Office (GSO), National Institute of Hygiene and Epidemiology (NIHE) [Vietnam], ORC Macro. Vietnam Population and AIDS Indicator Survey 2005. In. Calverton, Maryland, USA: GSO, NIHE, and ORC Macro; 2006. [Google Scholar]
- 36.Waturangi DE, Pradita N, Linarta J, Banerjee S. Prevalence and molecular characterization of vibrio cholerae from ice and beverages sold in Jakarta, Indonesia, using most probable number and multiplex PCR. J Food Prot 2012,75:651–659. doi: 10.4315/0362-028X.JFP-11-504 [DOI] [PubMed] [Google Scholar]
- 37.Weber JT, Mintz ED, Canizares R, Semiglia A, Gomez I, Sempertegui R, et al. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect 1994,112:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosewell A, Addy B, Komnapi L, Makanda F, Ropa B, Posanai E, et al. Cholera risk factors, Papua New Guinea, 2010. BMC Infect Dis 2012,12:287 doi: 10.1186/1471-2334-12-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabbani GH, Greenough WB 3rd. Food as a vehicle of transmission of cholera. J Diarrhoeal Dis Res 1999,17:1–9. [PubMed] [Google Scholar]
- 40.World Health Organization. Prevention and control of cholera outbreaks: WHO policy and recommendations. In. Geneva, Switzerland: WHO; Accessed June 23, 2016. [Google Scholar]
- 41.Kone-Coulibaly A, Tshimanga M, Shambira G, Gombe NT, Chadambuka A, Chonzi P, et al. Risk factors associated with cholera in Harare City, Zimbabwe, 2008. East Afr J Public Health 2010,7:311–317. [DOI] [PubMed] [Google Scholar]
- 42.Symaco LP. Education, poverty, malnutrition and famine. Colin Brock: Bloomsbury Academic; 2014. [Google Scholar]
- 43.Lamond E, Kinyanjui J. Cholera outbreak guidelines: Preparedness, prevention and control. Oxford, UK: Oxfam GB; 2012. [Google Scholar]
- 44.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque ASG, et al. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2008,2:e221 doi: 10.1371/journal.pntd.0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Characteristics of the sample with and without missing data (Table A). Cholera risk factors in Ben Tre, southern Vietnam, 2010 (Table B).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.