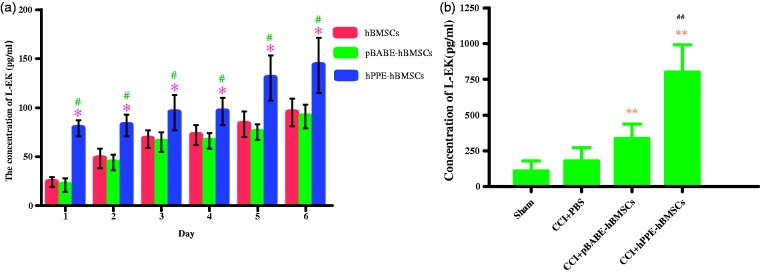
Figure 5.
Detecting the production of Leu-enkephalin (L-EK) released by human bone marrow stem cells (hBMSCs) modified with human proenkephin (hPPE) in vitro (a) and in vivo (b). In vitro, the supernatants in three groups such as the hBMSC, pBABE-hBMSC, and hPPE-hBMSC groups were collected for the concentration measurement of L-EK for six days after cells were seeding into culture plates. In vivo, the concentrations of L-EK in CSF in four groups such as the sham, CCI+PBS, CCI+pBABE-hBMSC, and CCI+hPPE-hBMSC groups on the 14th day after CCI operation were detected (n = 6). Enzyme immunoassay (ELISA) method was used for the measurement. Data are presented as the means ± SD, significance was defined as *P < 0.05 versus the hBMSC group, #P < 0.05 versus the pBABE-hBMSC group, **P < 0.05 versus the CCI+PBS group, ##P < 0.05 versus the CCI+pBABE-hBMSC group. CCI, chronic constriction injury; pBABE, a retroviral vector; hBMSCs, the hBMSC group; pBABE-hBMSCs, the pBABE-hBMSC group; hPPE-hBMSCs, the hPPE-hBMSC group; Sham, the Sham group; CCI+PBS, the CCI+PBS group; CCI+pBABE-hBMSCs, the CCI+pBABE-hBMSC group; CCI+hPPE-hBMSCs, the CCI+hPPE-hBMSC group.