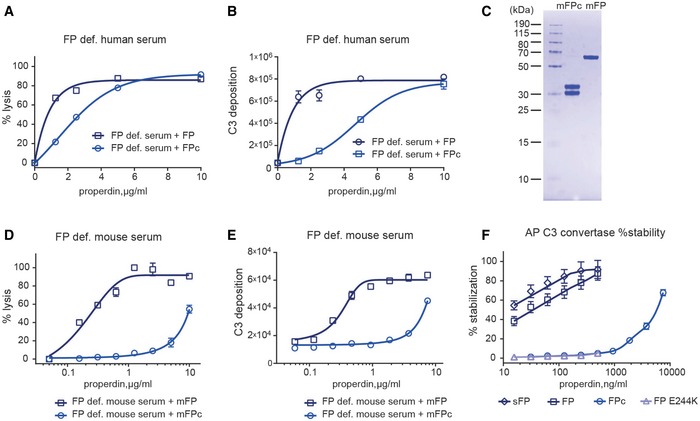
In a hemolysis assay with FP‐deficient serum using the AP initiating rabbit erythrocytes (Er), the addition of both recombinant human FP and FPc stimulated lysis.
The addition of FP and FPc to FP‐deficient serum also enhanced AP‐mediated C3b deposition on zymosan‐coated microtiter wells.
SDS–PAGE analysis of recombinant murine mFP and mFPc.
mFP and mFPc stimulated AP‐mediated lysis of Er in murine FP‐deficient serum.
Addition of mFP and mFPc to FP‐deficient murine serum also led to augmentation of C3b deposition in zymosan‐coated microtiter wells.
C3/C5 convertase stability assay performed with C3b‐covered sheep erythrocytes Es. The efficacy of serum‐derived sFP, FP, FP E244K, and FPc was measured as the % of the residual convertase on the erythrocyte surface compared to a reference without a 30‐min decay period. While recombinant FP and sFP stabilized the C3 convertase in a dose‐dependent manner, FP E244K and wild‐type FPc failed to stabilize the convertase, but with at very high FPc concentration some stabilization is observed.
Data information: Data on E
s hemolysis (panel F) are reported as means of triplicates, while E
r hemolysis and zymosan C3b deposition data (panels A, B, D, and E) are reported as means of duplicates. Error bars indicate SDs of the replicates.