Figure 5. Ataxin‐3 and RNF4 regulate ubiquitylation of MDC1.
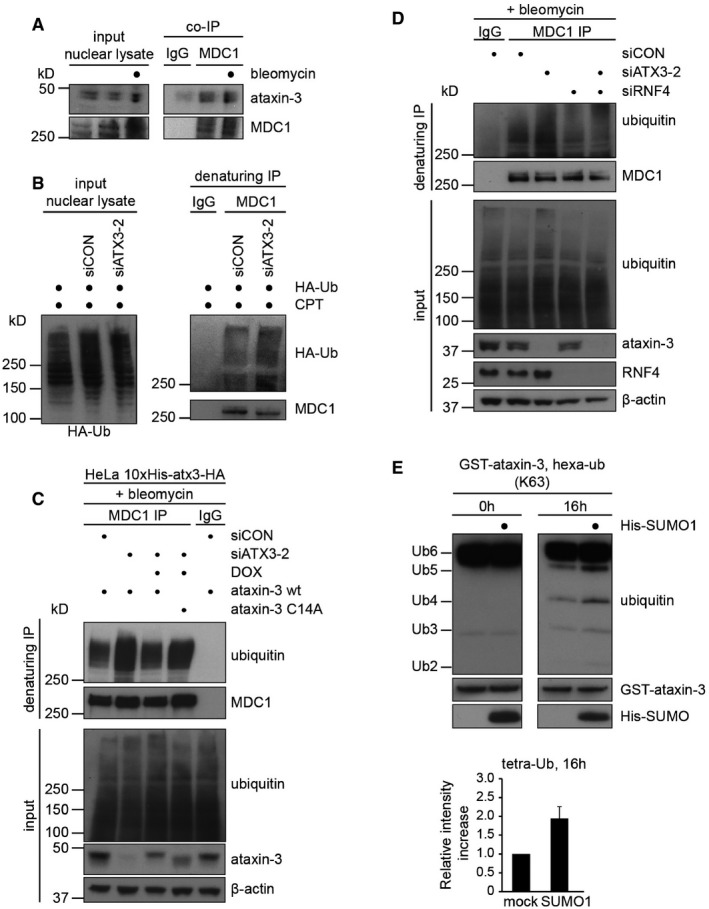
- MDC1 interacts with ataxin‐3. A co‐immunoprecipitation of endogenous MDC1 from U2OS cells was performed. DSBs were induced or not by 10 μg/ml bleomycin, and MDC1 (or IgG) was immunoprecipitated from nuclear lysates followed by Western blotting with indicated antibodies.
- U2OS cells were transfected with control or ataxin‐3 siRNA (48 h) and HA‐tagged ubiquitin (24 h). After induction of DNA damage with 20 μM camptothecin (CPT; 1 h), cells were fractionated in cytosolic and nuclear fractions and nuclei were re‐suspended in denaturing buffer. MDC1 (or IgG) was immunoprecipitated from nuclear lysates followed by Western blotting with indicated antibodies.
- Ubiquitylation assay of MDC1. HeLa cells expressing 10xHis‐ataxin‐3‐HA wild‐type or C14A cells were transfected with control or ataxin‐3 siRNA (48 h) and were induced by 1 μg/ml Dox (24 h) where indicated. After the induction of DNA damage, cells were re‐suspended in denaturing buffer. MDC1 (or IgG) was immunoprecipitated from lysates followed by Western blotting with indicated antibodies.
- As in (B) but U2OS cells were transfected with ataxin‐3 or RNF4 targeting siRNAs.
- The catalytic activity of GST‐ataxin‐3 was analyzed in vitro using K63‐linked hexa‐ubiquitin as a substrate. Reactions were stopped at 0 or 16 h. Where indicated, GST‐ataxin‐3 was pre‐incubated with recombinant His‐SUMO1 before addition of the ubiquitin substrate. The relative intensity of tetra‐ubiquitin product was quantified. Data are presented as mean ± SEM from three independent experiments.
