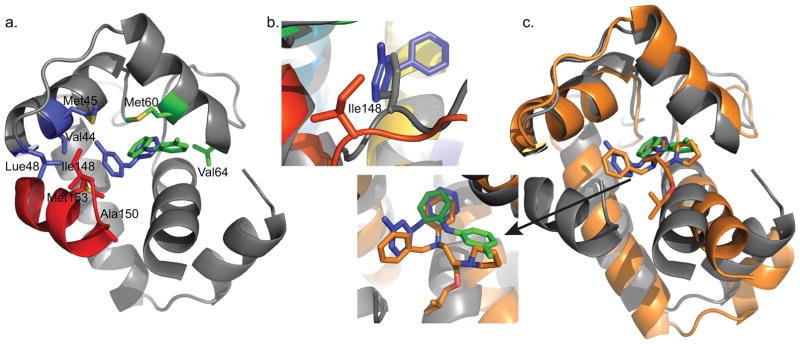
Figure 8.
a) cChimera residues with observed NOEs to the methyl-substituted ring of 3-mDPA. cChimera-3-mDPApeptide drug binding site, contacting Val44, Met45, and Leu48 of cNTnC are shown in blue, Ile148 and Met153 of cTnI are shown in blue. cChimera-3-mDPAsolvent exposed drug binding site, contacting Val64 and Met60, are shown in green. b) The sidechain of Ile148 is displaced when 3-mDPA binds at the peptide binding site (cChimera: grey, cChimera-3-mDPApeptide: red). c) The drug binding site of the cTnC-bepridil complex x-ray structure (orange), cChimera-3-mDPApeptide (cChimera: grey, 3-mDPA: blue) and cChimera-3-mDPAsolvent exposed (cChimera: grey, 3-mDPA: green). Note how steric clash of the bepridil isopropyl group pushes helices N and A away from the rest of the protein, increasing the size of the central cNTnC cavity in order to accommodate bepridil.