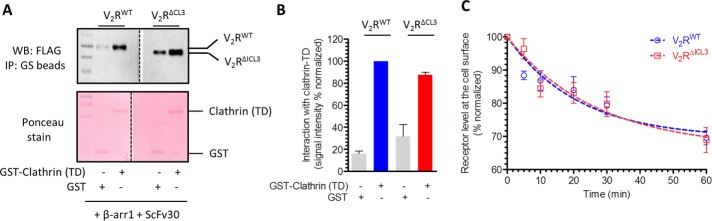
FIGURE 4:
V2RΔICL3-βarr1 complex binds clathrin, and V2RΔICL3 undergoes efficient endocytosis. (A) A coIP experiment reveals that V2RΔICL3-βarr1 complex (partially engaged) is able to interact with GST-clathrin-TD, similar to V2RWT-βarr1 complex (fully engaged). Here ScFv30 is used to stabilize V2R-βarr1 complexes to avoid any potential clash with clathrin binding. Purified GST is used as a negative control. A representative image of four independent experiments. (B) Densitometry-based quantification of coIP data in A. Data are normalized with respect to clathrin interaction with V2RWT-βarr1 complex (treated as 100%). (C) Agonist-induced endocytosis of V2RWT and V2RΔICL3 are comparable, suggesting that the inability of V2RΔICL3 to engage the core interaction with βarr does not affect receptor endocytosis. Here HEK-293 cells expressing either V2RWT or V2RΔICL3 were stimulated with 100 nM AVP (agonist) for indicated times, and the loss of surface receptor as measured by reactivity of HRP-coupled M2 antibody with N-terminal FLAG tag in whole-cell ELISA was used as a readout of receptor endocytosis. Data represent average ± SEM of six independent experiments each carried out in duplicate.