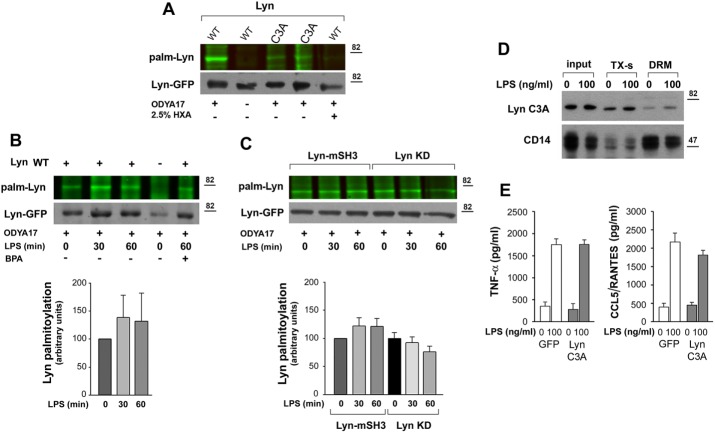
FIGURE 6:
Palmitoylated wild-type Lyn accumulates in the DRM fraction of RAW264 cells. (A–C) Palmitoylation of Lyn revealed by click chemistry. RAW264 transfectants were subjected to metabolic labeling with palmitic acid analogue ODYA17 or its carrier DMSO and left unstimulated or stimulated with 100 ng/ml LPS for 30–60 min. Cells were either lysed (A) or fractionated to obtain the DRM fraction (B, C). Subsequently Lyn-GFP was immunoprecipitated from the lysates or the DRM fraction and subjected to click chemistry with the fluorescent dye IRDye 800CW azide to reveal palmitoylation (top). The efficiency of immunoprecipitation was determined by blotting with anti-GFP antibody (bottom). (A) Labeling of Lyn WT and Lyn C3A with ODYA17 in unstimulated cells. HXA, samples treated with 2.5% hydroxylamine before electrophoresis. (B) Labeling of Lyn WT present in the DRM fraction during stimulation of cells with 100 ng/ml LPS. Control cells expressing GFP (–) and cells exposed to 125 μM BPA before labeling with ODYA17 and stimulation with LPS. (C) Labeling of Lyn-mSH3 and Lyn KD present in the DRM fraction during stimulation of cells with 100 ng/ml LPS. The level of Lyn palmitoylation was normalized against Lyn-GFP content in samples and is expressed in histograms relative to that in unstimulated cells. Mean ± SD of four or five experiments. (D) Distribution of Lyn C3A between Triton X-100–soluble (TX-s) and DRM fractions in unstimulated cells and cells exposed to 100 ng/ml LPS for 30 min. (E) Concentration of TNF-α and CCL5/RANTES in culture supernatants of GFP- or Lyn C3A–expressing cells measured with ELISA after 4 or 6 h of stimulation with 100 ng/ml LPS, respectively. Mean ± SD of two experiments run in triplicate.