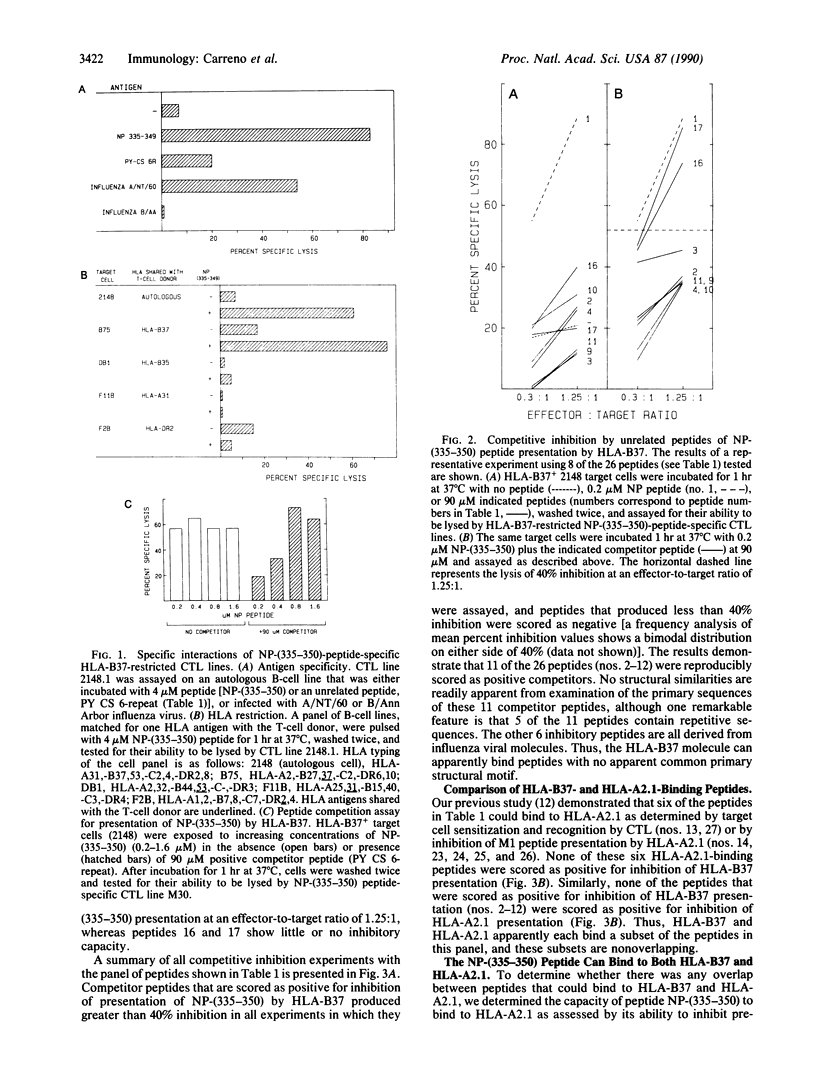
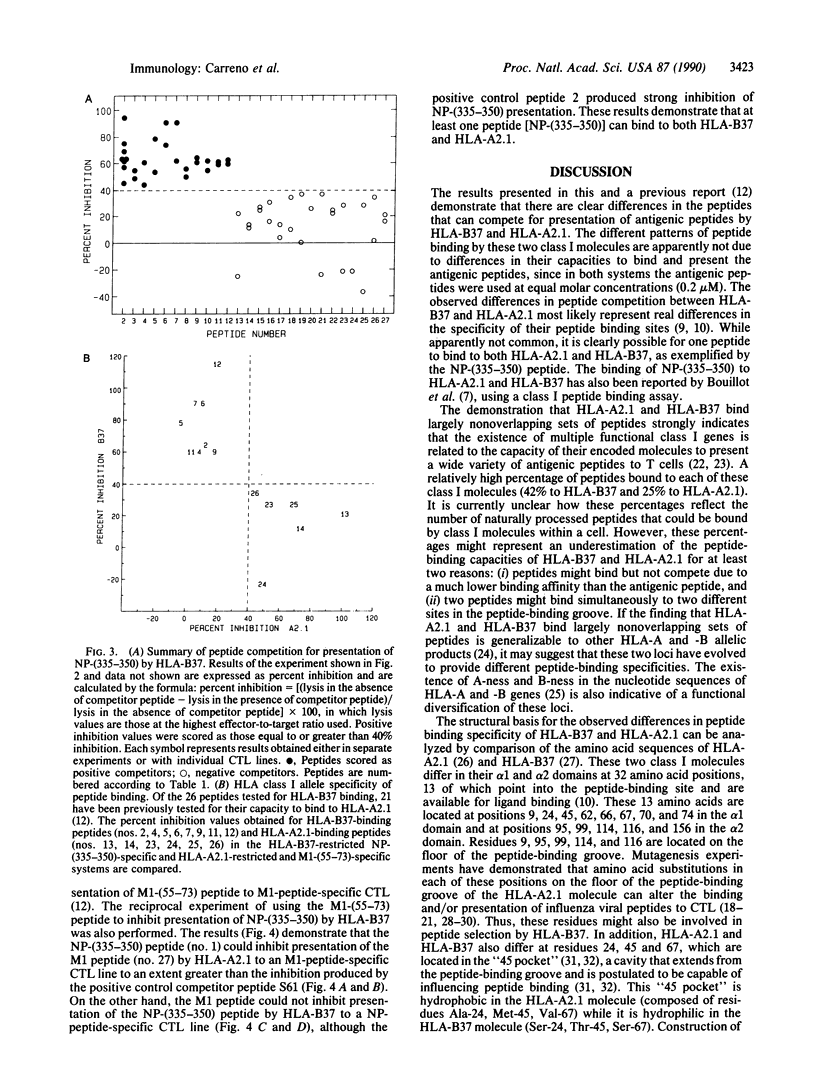
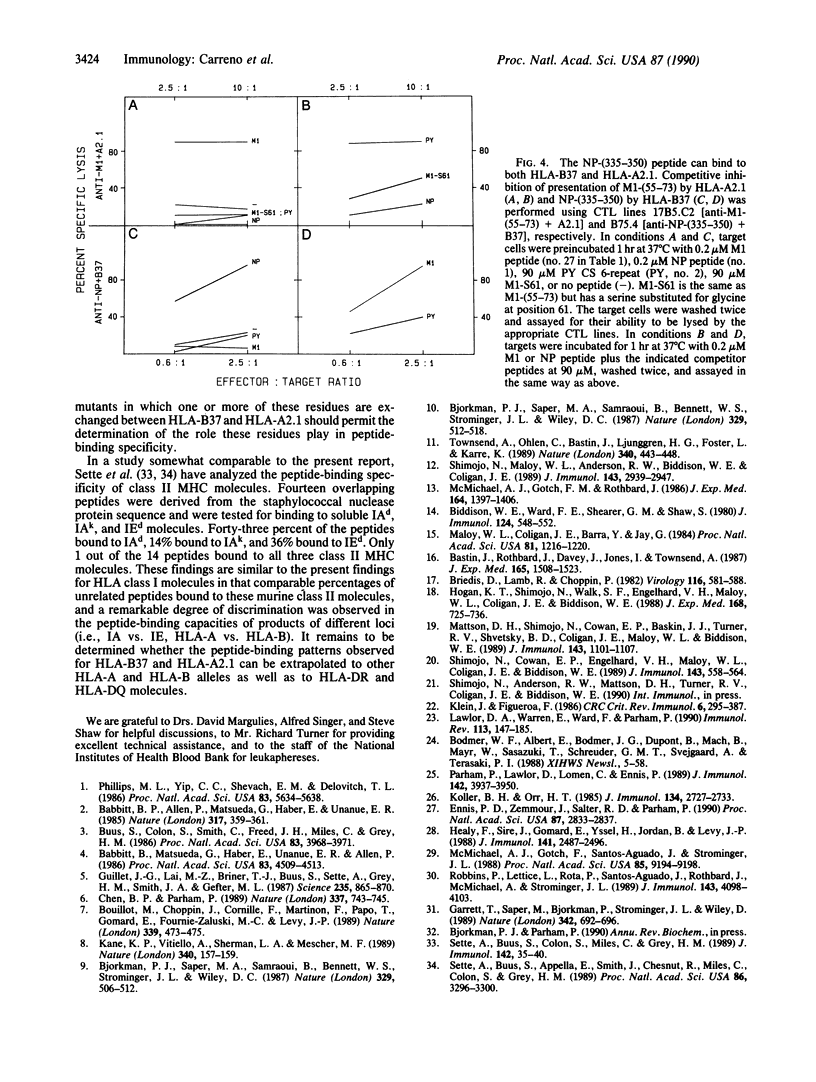
Abstract
T-cell recognition of peptides that are bound and presented by class I major histocompatibility complex molecules is highly specific. At present it is unclear what role class I peptide binding plays relative to T-cell receptor specificity in determination of immune recognition. A previous study from our group demonstrated that the HLA-A2.1 molecule could bind to 25% of the members of a panel of unrelated synthetic peptides as assessed by a functional peptide competition assay. To determine the peptide-binding specificity of another HLA class I molecule, we have examined the capacity of this panel of peptides to compete for the presentation of influenza virus nucleoprotein peptide NP-(335-350) by HLA-B37 to NP-peptide-specific HLA-B37-restricted cytotoxic T-lymphocyte lines. Forty-two percent of peptides tested were capable of inhibiting NP-(335-350) presentation by HLA-B37. Remarkably, none of these HLA-B37-binding peptides belong to the subset that was previously shown to bind to the HLA-A2.1 molecule. Only the NP-(335-350) peptide was capable of binding to both HLA-A2.1 and HLA-B37. These findings demonstrate that the peptide-binding specificities of HLA-B37 and HLA-A2.1 are largely nonoverlapping and suggest that, from the universe of peptides, individual HLA class I molecules can bind to clearly distinct subsets of these peptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Matsueda G., Haber E., Unanue E. R., Allen P. M. Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4509–4513. doi: 10.1073/pnas.83.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J., Rothbard J., Davey J., Jones I., Townsend A. Use of synthetic peptides of influenza nucleoprotein to define epitopes recognized by class I-restricted cytotoxic T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1508–1523. doi: 10.1084/jem.165.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddison W. E., Ward F. E., Shearer G. M., Shaw S. The self determinants recognized by human virus-immune T cells can be distinguished from the serologically defined HLA antigens. J Immunol. 1980 Feb;124(2):548–552. [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bouillot M., Choppin J., Cornille F., Martinon F., Papo T., Gomard E., Fournie-Zaluski M. C., Levy J. P. Physical association between MHC class I molecules and immunogenic peptides. Nature. 1989 Jun 8;339(6224):473–475. doi: 10.1038/339473a0. [DOI] [PubMed] [Google Scholar]
- Briedis D. J., Lamb R. A., Choppin P. W. Sequence of RNA segment 7 of the influenza B virus genome: partial amino acid homology between the membrane proteins (M1) of influenza A and B viruses and conservation of a second open reading frame. Virology. 1982 Jan 30;116(2):581–588. doi: 10.1016/0042-6822(82)90150-7. [DOI] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. P., Parham P. Direct binding of influenza peptides to class I HLA molecules. Nature. 1989 Feb 23;337(6209):743–745. doi: 10.1038/337743a0. [DOI] [PubMed] [Google Scholar]
- Ennis P. D., Zemmour J., Salter R. D., Parham P. Rapid cloning of HLA-A,B cDNA by using the polymerase chain reaction: frequency and nature of errors produced in amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2833–2837. doi: 10.1073/pnas.87.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Buus S., Sette A., Grey H. M., Smith J. A., Gefter M. L. Immunological self, nonself discrimination. Science. 1987 Feb 20;235(4791):865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Healy F., Sire J., Gomard E., Yssel H., Jordan B., Levy J. P. A study of functionally active amino acids involved in the interaction of HLA-A2 or HLA-A3 molecules with cytolytic T lymphocytes. J Immunol. 1988 Oct 1;141(7):2487–2496. [PubMed] [Google Scholar]
- Hogan K. T., Shimojo N., Walk S. F., Engelhard V. H., Maloy W. L., Coligan J. E., Biddison W. E. Mutations in the alpha 2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza virus matrix peptide. J Exp Med. 1988 Aug 1;168(2):725–736. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane K. P., Vitiello A., Sherman L. A., Mescher M. F. Cytolytic T-lymphocyte response to isolated class I H-2 proteins and influenza peptides. Nature. 1989 Jul 13;340(6229):157–159. doi: 10.1038/340157a0. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F. Evolution of the major histocompatibility complex. Crit Rev Immunol. 1986;6(4):295–386. [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Lawlor D. A., Warren E., Ward F. E., Parham P. Comparison of class I MHC alleles in humans and apes. Immunol Rev. 1990 Feb;113:147–185. doi: 10.1111/j.1600-065x.1990.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E., Barra Y., Jay G. Detection of a secreted form of the murine H-2 class I antigen with an antibody against its predicted carboxyl terminus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1216–1220. doi: 10.1073/pnas.81.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson D. H., Shimojo N., Cowan E. P., Baskin J. J., Turner R. V., Shvetsky B. D., Coligan J. E., Maloy W. L., Biddison W. E. Differential effects of amino acid substitutions in the beta-sheet floor and alpha-2 helix of HLA-A2 on recognition by alloreactive viral peptide-specific cytotoxic T lymphocytes. J Immunol. 1989 Aug 15;143(4):1101–1107. [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986 Nov 1;164(5):1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Santos-Aguado J., Strominger J. L. Effect of mutations and variations of HLA-A2 on recognition of a virus peptide epitope by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9194–9198. doi: 10.1073/pnas.85.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Lawlor D. A., Lomen C. E., Ennis P. D. Diversity and diversification of HLA-A,B,C alleles. J Immunol. 1989 Jun 1;142(11):3937–3950. [PubMed] [Google Scholar]
- Phillips M. L., Yip C. C., Shevach E. M., Delovitch T. L. Photoaffinity labeling demonstrates binding between Ia molecules and nominal antigen on antigen-presenting cells. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5634–5638. doi: 10.1073/pnas.83.15.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. A., Lettice L. A., Rota P., Santos-Aguado J., Rothbard J., McMichael A. J., Strominger J. L. Comparison between two peptide epitopes presented to cytotoxic T lymphocytes by HLA-A2. Evidence for discrete locations within HLA-A2. J Immunol. 1989 Dec 15;143(12):4098–4103. [PubMed] [Google Scholar]
- Sette A., Buus S., Appella E., Smith J. A., Chesnut R., Miles C., Colon S. M., Grey H. M. Prediction of major histocompatibility complex binding regions of protein antigens by sequence pattern analysis. Proc Natl Acad Sci U S A. 1989 May;86(9):3296–3300. doi: 10.1073/pnas.86.9.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Buus S., Colon S., Miles C., Grey H. M. Structural analysis of peptides capable of binding to more than one Ia antigen. J Immunol. 1989 Jan 1;142(1):35–40. [PubMed] [Google Scholar]
- Shimojo N., Cowan E. P., Engelhard V. H., Maloy W. L., Coligan J. E., Biddison W. E. A single amino acid substitution in HLA-A2 can alter the selection of the cytotoxic T lymphocyte repertoire that responds to influenza virus matrix peptide 55-73. J Immunol. 1989 Jul 15;143(2):558–564. [PubMed] [Google Scholar]
- Shimojo N., Maloy W. L., Anderson R. W., Biddison W. E., Coligan J. E. Specificity of peptide binding by the HLA-A2.1 molecule. J Immunol. 1989 Nov 1;143(9):2939–2947. [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]


