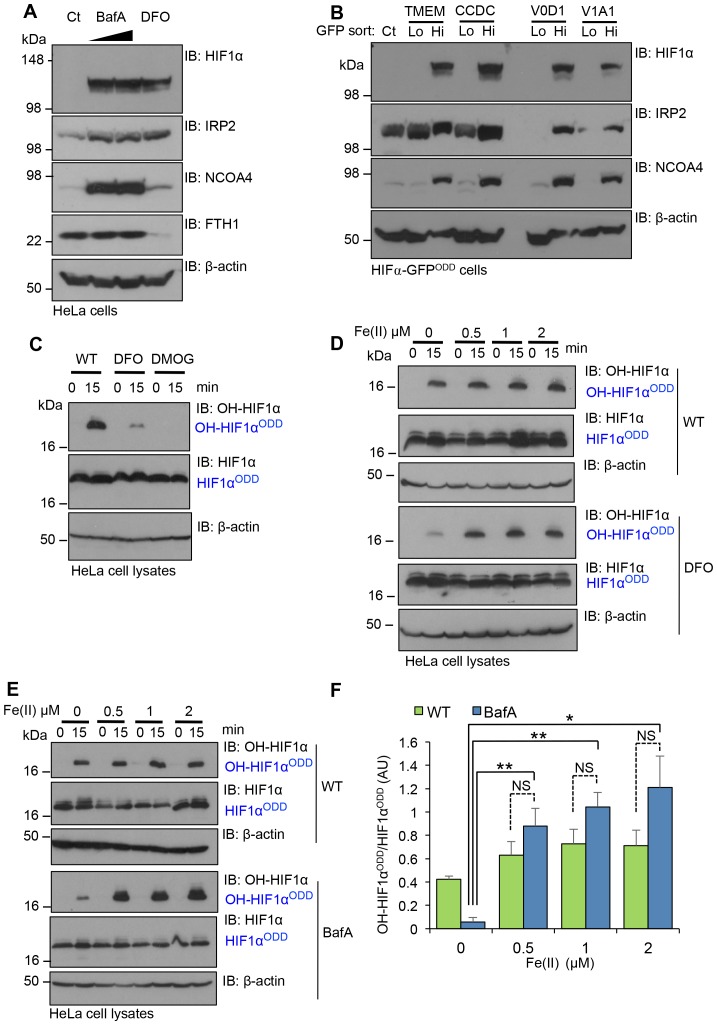
Figure 8. Disrupting V-ATPase activity decreases intracellular iron levels.
(A, B) V-ATPase inhibition leads to intracellular iron depletion. (A) HeLa cells were treated with BafA (10 nM or 100 nM) or 100 µM DFO for 24 hr. HIF1α, IRP2, NCOA4 and ferritin (ferritin heavy chain 1, FTH1) levels were measured by immunoblot. (B) HIF1α-GFPODD reporter cells transduced with Cas9 and sgRNA targeting V-ATPase components (TMEM199, CCDC115, ATP6V0D1 and ATP6V1A1) were sorted into GFPLOW (Lo) and GFPHIGH (Hi) populations as described. The lysates were immunoblotted for HIF1α, IRP2, or NCOA4. β-actin served as a loading control. (C) Iron chelation prevents HIF1α hydroxylation. In vitro prolyl hydroxylation of the HIF1αODD protein following incubation with lysates from WT or DFO treated lysates (100 µM for 24 hr) as previously described. DMOG served as a control for PHD inhibition. (D–F) In vitro hydroxylation of PHD activity in DFO or BafA treated lysates supplemented with ferrous iron. Lysates from control, DFO (D) or BafA (E) treated HeLa cells were extracted as previously described, incubated with the HIF1αODD protein, and supplemented with increasing concentrations of iron chloride (FeCl2, Fe(II)). Prolyl-hydroxylated HIF1αODD levels were visualised by immunoblot and quantified by densitometry for the BafA treated lysate (F) (n = 3). Values are mean±SEM. *p<0.05, **p<0.01 Fe(II) compared to no treatment in BafA treated cells. NS=not significant.