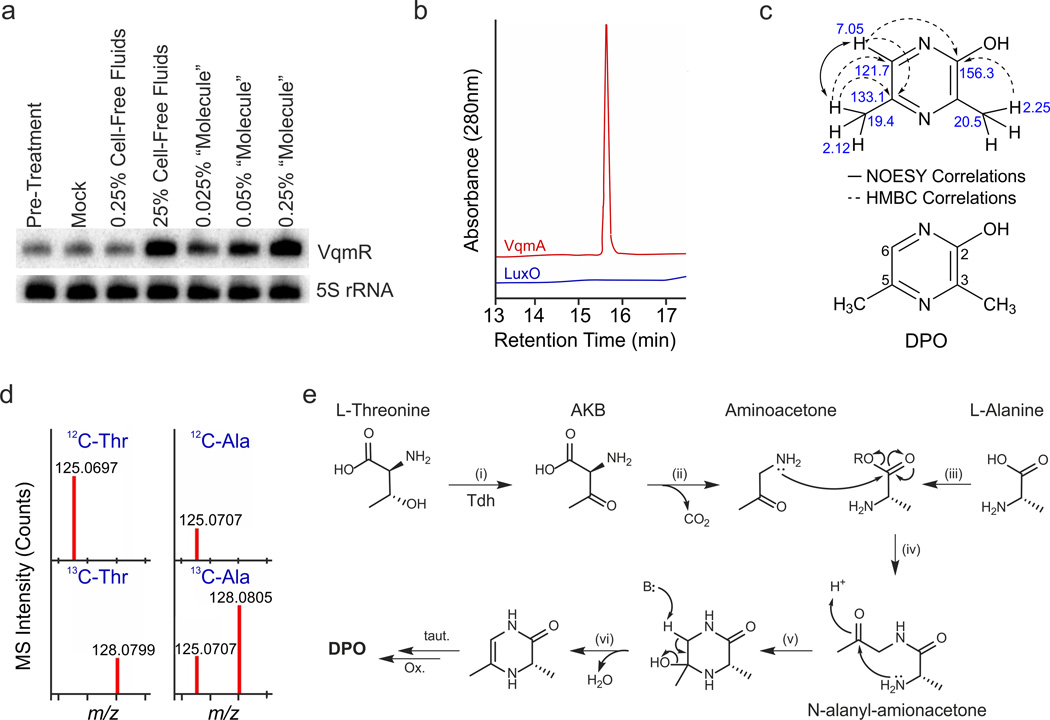
Fig. 4. Identification and proposed biosynthesis of DPO.
(A) The V. cholerae Δtdh mutant was grown in M9 medium to an OD600 of 1.0 and treated with 0.25% or 25% (final conc.) of wild-type V. cholerae cell-free culture fluids. Alternatively, the cells were treated with the “molecule” released from the 6XHIS::VqmA protein (0.025%, 0.05%, and 0.25% final conc.). Ten min after addition of each preparation, VqmR levels were assessed by Northern Blot. Pre-treatment: collected prior to treatment. Mock: no addition. 5S rRNA served as loading control. Source files with blots showing the full data can be found in Supplementary Fig. 8C. (B) HPLC-Qtof-MS analysis of compounds released from purified VqmA (red trace) and purified LuxO control (blue trace). Shown are elution profiles monitored at 280 nm, with the peak observed in the VqmA sample corresponding to DPO. (C) NOESY and HMBC correlations (top) used to solve the structure of DPO (bottom). The 1H and 13C chemical shifts (top) and the numbering scheme (bottom) for DPO are shown. (D) Mass-spectra of DPO purified from wild-type V. cholerae grown with supplemental L-Thr, uniformly-labeled 13C4-L-Thr, L-Ala, and uniformly labeled 13C3-L-Ala. The presence of a low level of 12C-DPO following supplementation with 13C3-L-Ala indicates that residual 12C-Ala, generated from the carbon-source glucose (via pyruvate), is incorporated into DPO. (E) Proposed biosynthesis of DPO. Tdh catalyzes the oxidation of Thr to AKB (i), which spontaneously decarboxylates to aminoacetone (ii). Ala, activated by an unidentified enzyme (R = phosphoryl, adenylyl, or CoA group) (iii), is condensed with aminoacetone (iv), followed by intramolecular cyclization (v), dehydration (vi), spontaneous tautomerization and oxidation, to yield DPO. We name DPO compound 1 and N-alanyl-aminoacetone compound 2 in the online chemical compound information file.