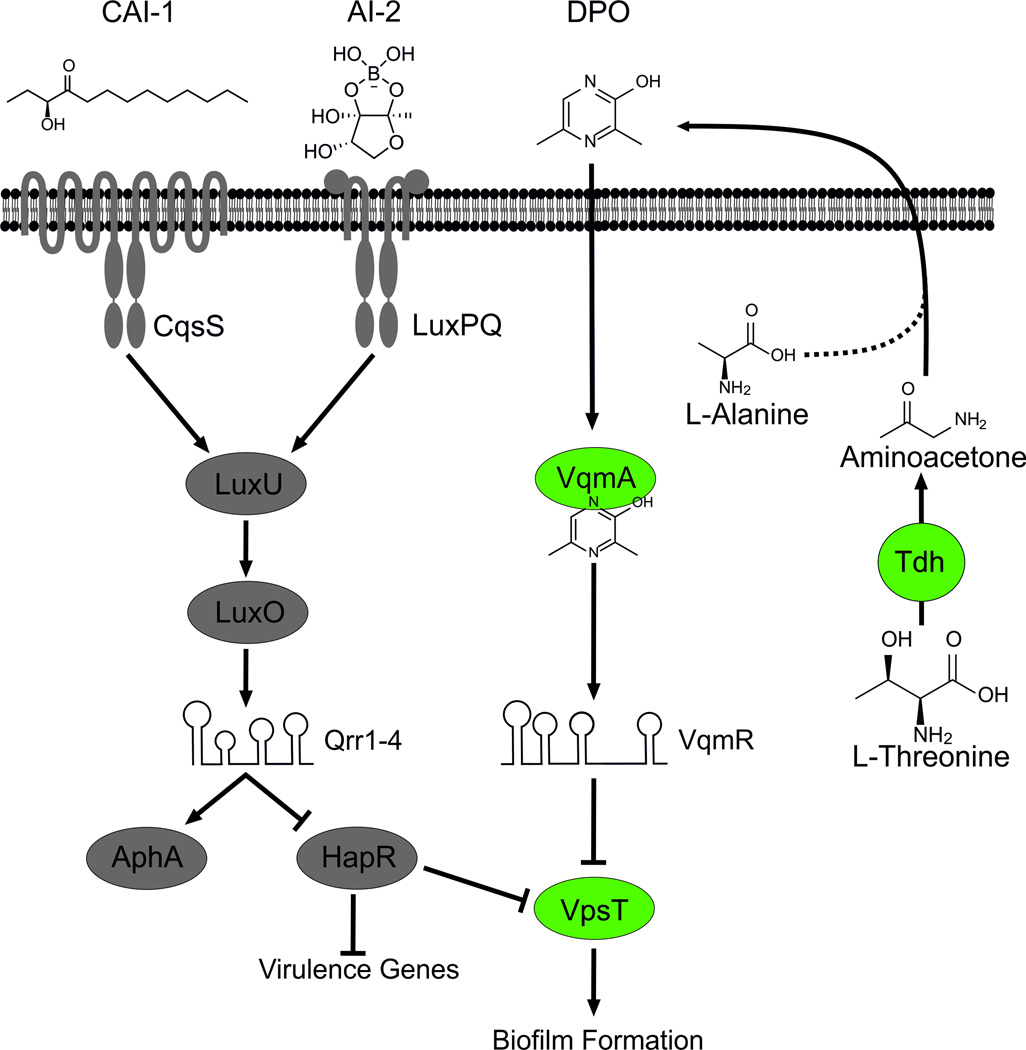
Fig. 6. DPO-dependent and independent QS pathways in V. cholerae.
Left (grey): The two previously characterized canonical QS pathways in V. cholerae. CAI-1 and AI-2 are detected by the CqsS and LuxPQ receptors, respectively. At low cell density, CqsS and LuxPQ function as kinases to phosphorylate LuxU. LuxU~P shuttles the phosphate to LuxO, and LuxO~P activates transcription of genes encoding the Qrr1–4 sRNAs. The Qrr sRNAs post-transcriptionally repress hapR and activate aphA promoting virulence gene expression and biofilm formation. At high cell density, binding of the CAI-1 and AI-2 autoinducers to CqsS and LuxPQ, respectively, converts them from kinases to phosphatases, reversing the phosphorylation cascade. Consequently, production of Qrr1–4 ceases. Under this condition, aphA translation is not activated and hapR translation is de-repressed. HapR represses genes required for virulence. HapR also represses biofilm formation via negative regulation of vpsT. Right (green): The DPO-dependent QS pathway in V. cholerae. DPO is produced from threonine catabolism, and DPO production requires the Tdh (threonine dehydrogenase) enzyme. We propose that the product, aminoacetone, is condensed with alanine to form DPO. Additional enzymes may be involved but they are not yet identified. DPO or a DPO precursor is released into the environment. Extracellular DPO, acting as an autoinducer, and possibly intracellularly produced DPO, binds to the VqmA receptor, which activates it. The VqmA-DPO complex activates transcription of the vqmR gene encoding the VqmR sRNA. VqmR inhibits biofilm formation by repressing translation of vpsT.