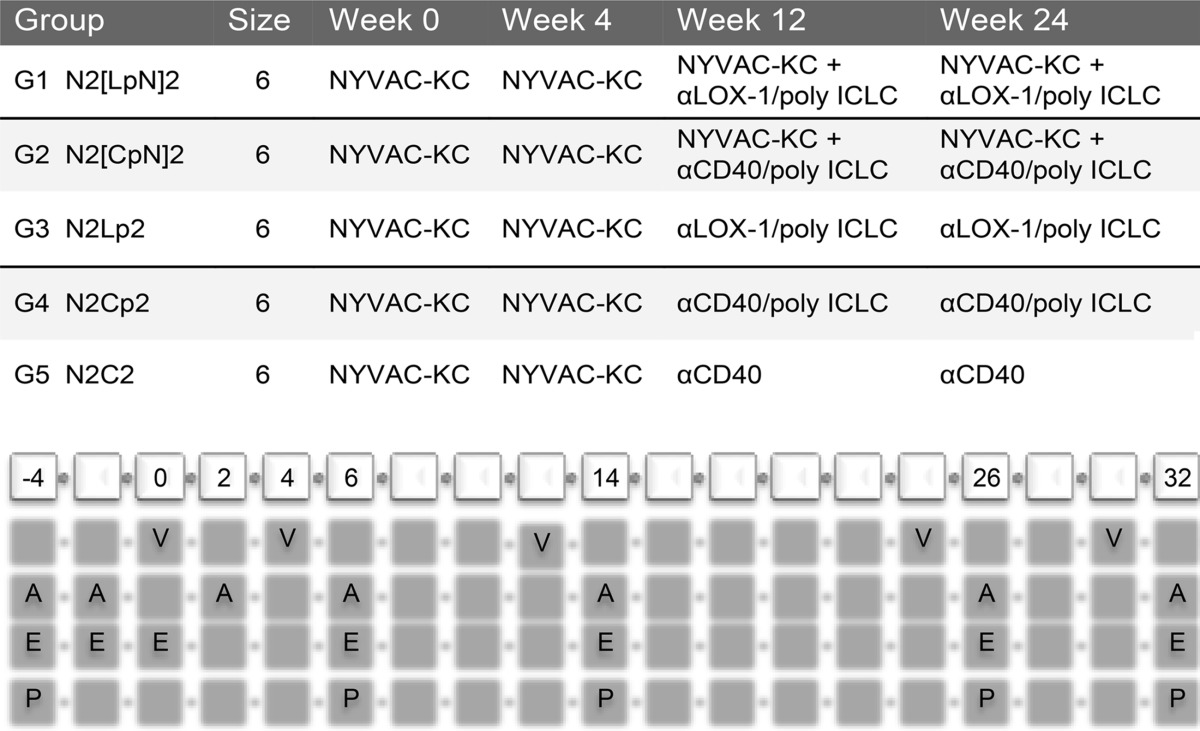
TABLE 1.
Study design for testing antigenicity of αLOX-1.Env gp140 and αCD40.Env gp140 fusion proteins in NHPsa
Shown are the immunization regimens used in this study. The reference codes for the five vaccination groups are as follows: N, NYVAC-KC; L, anti-LOX-1.Env gp140; p, poly-ICLC; 2, administered twice in sequence. NYVAC-KC administration was intramuscular (i.m.), DC-targeting protein administration was intradermal (i.d.), and poly-ICLC administration was subcutaneous (s.c.) in proximity to the protein. The cells at the bottom are in biweekly increments starting at week −4 through week 32. V, vaccination dates; A, serum collection for antibody response determination; E, blood collection for IFN-γ ELISPOT measurements; P, frozen PBMC preparation for antigen-specific T cell analysis via flow cytometry including intracellular cytokine staining.