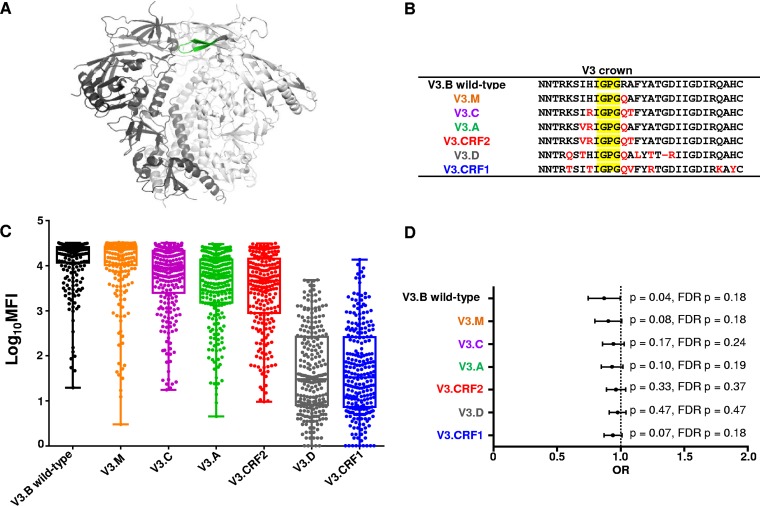
FIG 1.
Breadth of plasma V3-specific IgG responses of HIV-1-infected mothers from the WITS cohort. Maternal plasma linear V3-specific IgG responses were tested against a multiclade consensus linear V3 peptide panel. (A) HIV Env gp120 with the V3 loop in green (29). (B) Differences in amino acid residues across the multiclade consensus V3 peptides compared to the V3.B consensus peptide (red); the V3 loop crown is shaded in yellow. (C) Clade-specific maternal V3-specific IgG responses (n = 248) measured by BAMA. The horizontal lines indicate the medians, and the boxes depict 25 to 75% MFI against each peptide. The error bars depict ranges. The V3-specific MAb CH22 was used as a positive control (mean MFIs at 5 μg/ml, 27,808, 28,309, 25,991, 23,476, 26,317, 2,301, and 826 for V3.B, V3.M, V3.C, V3.A, V3.CRF2, V3.D, and V3.CRF1, respectively). Normal human serum was used as a negative control (mean MFI at 1:500 dilution, 100). (D) Odds ratio plot of maternal clade-specific V3-specific IgG binding responses and MTCT risk. Raw (p) and false-discovery rate (FDR p) P values are reported.