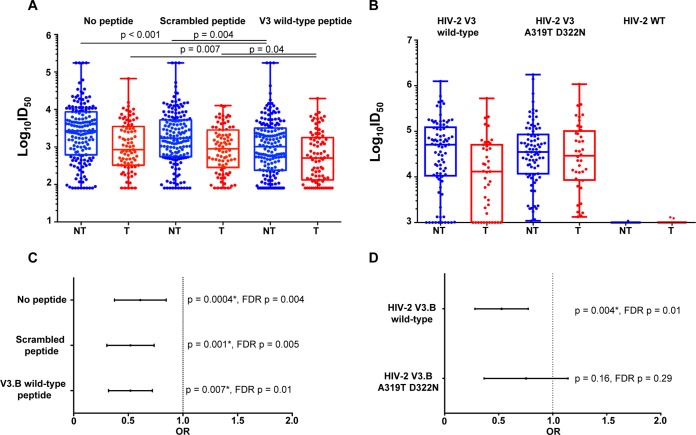
FIG 5.
Maternal plasma linear V3-specific neutralizing responses and MTCT risk. (A) Maternal neutralizing responses to HIV-1 SF162 in nontransmitting and transmitting women in the presence of no peptide, a scrambled peptide, and a V3.B wild-type linear peptide, with P values from a Mann-Whitney test. (B) Maternal plasma linear V3-specific neutralizing responses against HIV-2 V3.B wild type and a HIV-2 V3.B A319T D322N mutant. The V3-specific MAb CH22 was used as a positive control, and normal human serum was used as a negative control. (C) Odds ratio plot of maternal tier 1 virus-neutralizing responses to HIV-1 SF162 in the presence of no peptide and scrambled and V3.B wild-type peptide and MTCT risk. (D) Odds ratio plot of maternal V3-specific neutralizing responses against HIV-2 V3.B wild-type and HIV-2 V3.B A319T D322N chimeric viruses and MTCT risk. As the analysis was designed as a secondary, immune correlate analysis, significant raw P values are indicated by asterisks (P < 0.05).