Abstract
This year marks the 50th anniversary of the discovery of the Australia antigen (Blumberg, Alter and Visnich, 1965), which in 1968 was identified to be the hepatitis B virus (HBV) surface antigen. Even though several antiviral medications have been in use for the management of chronic HBV infection for more than 20 years, sustained clearance of HBsAg, similar to the sustained viral response (SVR) or cure in chronic hepatitis C (HCV), occurs in only a minority of treated patients. Moreover, even after 10 years of effective suppression of HBV viremia with current therapy, there is only a 40-70% reduction in deaths from liver cancer. Recent success in developing antivirals for hepatitis C that are effective across all genotypes has renewed interest in a similar cure for chronic HBV infection. In this article, we review a wave of newly identified drug targets, investigational compounds and experimental strategies that are now under clinical evaluation or in preclinical development. The paper forms part of a symposium in Antiviral Research on “An unfinished story: From the discovery of the Australia antigen to the development of new curative therapies for hepatitis B.”
Keywords: hepatitis B virus, chronic hepatitis B, antiviral therapy, clinical trials
This year marks the 50th anniversary of the discovery of the Australia antigen (Blumberg, Alter and Visnich, 1965), which in 1968 was identified to be the hepatitis B virus (HBV) surface antigen. Even though several antiviral medications have been in use for the management of chronic HBV infection for more than 20 years, sustained clearance of HBsAg, similar to the sustained viral response (SVR) or cure in chronic hepatitis C (HCV), occurs in only a minority of treated patients. Moreover, even after 10 years of effective suppression of HBV viremia with current therapy, there is only a 40-70% reduction in deaths from liver cancer. Recent success in developing combination antiviral therapies for hepatitis C that are effective across all genotypes has renewed interest in a similar cure for chronic HBV infection.
This article introduces a symposium in Antiviral Research on “An unfinished story: From the discovery of the Australia antigen to the development of new curative therapies for hepatitis B.” This collection of some 15 invited papers describes a wave of newly identified drug targets, investigational compounds and experimental strategies that are now under clinical evaluation or in preclinical development. In this article, we provide a general overview of these new approaches, and refer readers to papers in the symposium in which additional information on each type of novel therapy can be found.
1. INTRODUCTION
More than 350 million people are chronically infected with hepatitis B virus (HBV), with some 600,000 deaths per year attributed to the virus (El-Serag and Rudolph, 2007; Kanwal et al., 2015). Chronic HBV infection is associated with significant morbidity and mortality, secondary to acute and chronic hepatitis, fibrosis, cirrhosis, end-stage liver diseases and primary hepatocellular carcinoma (HCC) (see forthcoming review by Gish et al. in this symposium). It is estimated that, if left untreated, approximately 15-25% of chronically infected individuals would develop liver cirrhosis and HCC, after decades of infection (Block et al., 2007; Block et al., 2003). Although the precise mechanisms involved in the virus-mediated pathology are not completely known, it is generally assumed that suppression of HBV replication and antigen production is beneficial. Indeed, there is now considerable evidence that suppression of HBV DNA replication can arrest, and even reverse liver fibrotic disease and decrease the incidence of HCC (Bedossa, 2015; Lok, 2004; Lok and McMahon, 2009).
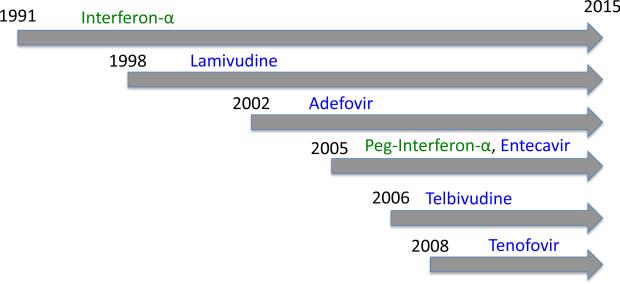
The first drug to be approved for managing chronic HBV infection was interferon-α 2b (Intron A®), in 1991. Since then, seven drugs have been approved, the most recent being tenofovir disoproxil fumarate (Viread®) in 2008 (Hoofnagle et al., 2007) (Figure 1). Broadly, these drugs can be classified as either host-targeting antivirals (HTA) or direct-acting antivirals (DAA) (Figure 2). HTAs target host gene products, while DAAs target viral gene products.
Figure 1.
Time-line of approval of hepatitis B therapeutics by the US Food and Drug Administration. Green: immunodulators (interferons); Blue: Direct-acting antivirals (polymerase inhibitors).
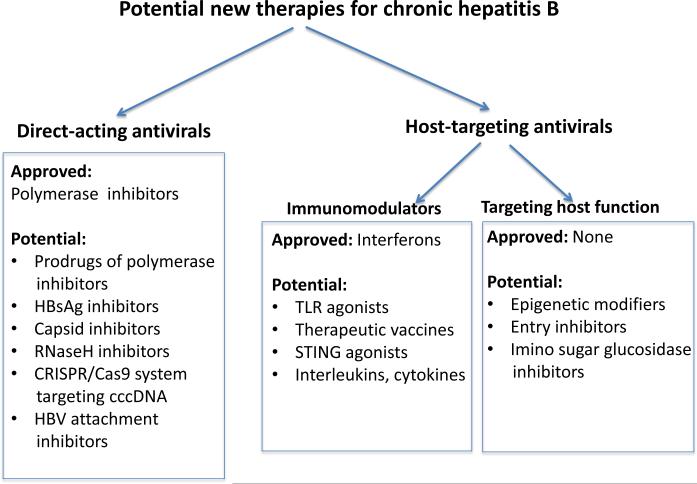
Figure 2.
Categorization of therapeutics for management of chronic HBV infection. Direct-acting antivirals (DAAs) interfere with a specific step in viral replication. Host-targeted antivirals (HTA) inhibit viral replication by modifying host cell function.
To date, the only approved HTAs are the interferons, and there are two approved for use in the United States: interferon-α2b (Intron A®) and peginterferon-α2a (Pegasys®). The pegylated form of interferon-α is considered to be an improvement over the non-pegylated form, with a decreased renal clearance rate, longer half-life, and increased bioavailability, thereby reducing the number of times per week that it needs to be injected, to once weekly. However, pegylated interferon-α showed no improvement in the side-effects profile caused by the first-generation interferon-α (Chae and Hann, 2007).
There are currently five approved DAAs for chronic hepatitis B in the US, all of which are nucleot(s)ide analogues: lamivudine (Epivir-HBV®), adefovir dipivoxil (Hepsera®), entecavir (Baraclude®), telbivudine (Tyzeka™) and tenofovir disoproxil fumarate (Viread®). All of them inhibit the reverse transcriptase/polymerase activity, resulting in a decrease in viral replication as measured by reductions in serum HBV DNA (see review by Gish et al. in this symposium). Use of the current therapeutics has been widely reviewed (Hoofnagle et al., 2007; Liaw et al., 2008; Lok and McMahon, 2009). Many other DAAs and HTAs can be contemplated (Figure 3).
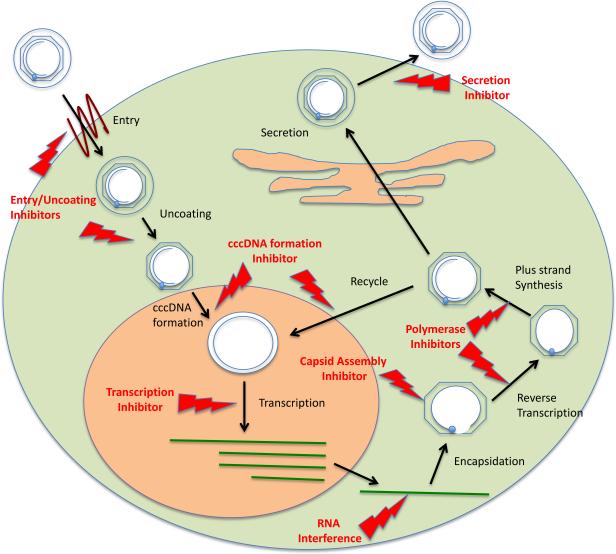
Figure 3.
Major intracellular steps in the HBV life cycle for which DAAs or HTAs could be developed. Each step is discussed in this review, and novel therapies targeting many of them are the subject of articles in this symposium.
Is a cure for hepatitis B possible? Hepatitis C patients are now routinely cured of their chronic viral infection with the use of combination all-oral DAA regimens or DAA regimens in combination with pegylated interferon, with or without ribavirin. Current HCV combination regimens can achieve an off-therapy sustained viral response at 12 weeks post completing therapy (SVR 12) in ~ 90-100% of patients, across all genotypes and all stages of chronic hepatitis C (Hoofnagle and Sherker, 2014). It is often reasoned that management of chronic HBV infection is likely to be more refractory than chronic HCV infection, because HBV persists with a nuclear phase, and can reactivate, even after decades of indolence (Hoofnagle, 2009; Lok et al., 2012; Seto et al., 2014) or with immunosuppression. This is mainly due to the presence of covalently closed circular DNA (cccDNA) of HBV present in the nucleus of infected hepatocytes (see forthcoming review by Guo and Guo in this symposium). cccDNA is a highly stable structure that acts as a minichromosome for all HBV transcripts. HBV also appears to be less responsive than HCV to interferons (Aspinall et al., 2011; Brouwer et al., 2015; Chan et al., 2011; Locarnini, 2004). However, the most effective management of chronic HCV infection today is with interferon-free, all-oral DAA regimens (Afdhal et al., 2014; Curry et al., 2015; Kowdley et al., 2014).
Complete suppression of the HBV polymerase should, in theory, reduce viremia and intrahepatic levels of replicative forms of HBV DNA to zero, and even cccDNA should be eliminated, as the infected cells are eventually replaced (Block et al., 2013). Therefore, according to this model, people with chronic HBV infection should be cured with DAAs alone. However, this has not been the case with most patients. Perhaps complete inhibition of the HBV polymerase has not been routinely achieved, since the degree of suppression of viral replication has been inadequate. We note that the most impressive and curative hepatitis C suppression has not been achieved with single DAAs, but with powerful combinations that target different steps in the viral replication cycle (Kowdley et al., 2014; Pawlotsky, 2014). However, combinations for HBV that are curative or even reliably superior to monotherapy have not been demonstrated with current medications, and even the most effective currently available DAAs for HBV do not drive intrahepatic levels of HBV DNA down by more than 2 log10 (despite 5-10 log10 reductions in serum viremia) (Block et al., 2013; Lau et al., 2005; Lee et al., 2013; Pellicelli et al., 2008; Werle-Lapostolle et al., 2004; Yang et al., 2012).
The sustained, and substantial, intra-hepatic HBV DNA levels, even after more than a year of > 5 log10 reductions in viremia, suggest that the current polymerase inhibitors are not inhibiting the enzyme by more than 99%. Thus, although these reductions in viremia are very impressive, they are still incomplete. Enzyme inhibition principles dictate that every additional ten-fold suppression in polymerase activity will require significantly greater amounts of inhibitor. However increasing the amounts of currently used polymerase inhibitors may not be safely tolerated in humans. The current polymerase inhibitors were optimized, and dosed to reduce serum HBV DNA levels to below the limits of detection, but not intra-hepatic viral DNA. Perhaps, if there were more potent polymerase inhibitors, or alternative means to decrease intra-hepatic levels of HBV DNA, including cccDNA, it would be possible to cure people with chronic HBV infection, with DAAs, in the same way that HCV is currently cured. In this review, we consider this possibility and identify several DAAs that might serve as alternatives or be complimentary, as part of new combination regimens, to the current portfolio of approved single-agent medications.
But how effective are current therapies for chronic hepatitis B? Clinically, chronically infected individuals have been divided into two groups, based on the presence or absence of the HBV gene product, HBeAg, which is derived from the capsid protein gene (Ganem and Prince, 2004; Hoofnagle et al., 2007). Although the significance of these subcategories continues to be debated, HBeAg-positive individuals generally have greater serum viral loads. Clinical trials have customarily aimed to sero-convert HBeAg-positive individuals into becoming HBeAg-negative, and HBe-antibody (HBeAb) positive (Gish et al., 2010; Hoofnagle et al., 2007). Both interferons and polymerase inhibitors are able to achieve HBeAg/Ab sero-conversion in approximately one-third of cases (Gordon et al., 2014; Lok and McMahon, 2009). These also represent the subset of those experiencing viremia reduction, which is routinely achieved in almost everyone treated with polymerase inhibitors. Indeed, at least 90% will have serum HBV DNA levels reduced by 4-6 orders of magnitude, often reaching undetectable or nearly undetectable levels by current methods (Liaw and Crawford, 1999; Liaw et al., 2004; Zeisel et al., 2015). However, reductions of intrahepatic viral DNA are far more modest, usually only 2 log10, even after two years of treatment, and this may be responsible for sequelae of virus reactivation and promote persistent liver disease (Lok, 2011; Zoulim and Durantel, 2015; Zoulim and Locarnini, 2009).
Currently, the treatment of chronic hepatitis B must be life-long for the majority of patients, because virus rebound occurs, often within weeks to months after cessation of treatment (Cho et al., 2014; Yuen and Lai, 2011). More concerning, even after 5-10 years of viremia suppression, the reduction in deaths due to liver disease is only 40-70% (Arends et al., 2014; Chang et al., 2006; Gordon et al., 2014; Lok, 2011; Yapali et al., 2014). Finally, current recommendations advise therapy only for those with elevated viremia and serum transaminases, leaving at least half of patients at significant risk of liver disease without any medical options (EASL, 2012; Liaw et al., 2012; Lok and McMahon, 2009; Uribe et al., 2014; Yapali et al., 2014). New approaches are clearly needed.
2. A new wave of hepatitis B therapies
In addition to the currently approved interferons and nucleot(s)ide inhibitors, there is the possibility of developing new agents to inhibit viral replication, with mechanisms of action that have not yet been explored. As shown in Figure 3, a number of critical steps in the HBV life cycle can potentially be targeted to decrease HBV replication. These steps use both host pathways/proteins and HBV-specific proteins, so that new HTAs and DAAs could be developed. In the following sections, we briefly profile several promising investigational HTAs and DAAs under clinical development, many of which target steps in the HBV life cycle not previously exploited. We also refer readers to symposium articles in which each novel approach to therapy is reviewed. Readers may also wish to see a review by Liang et al (Hepatology, accepted)
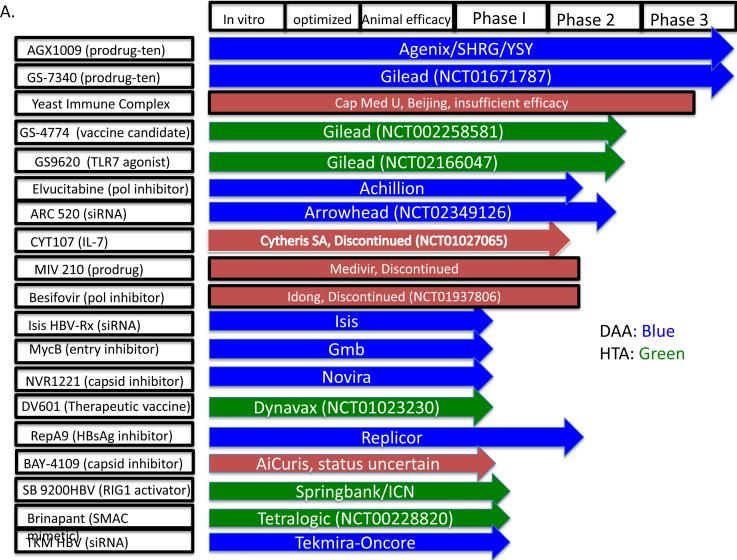
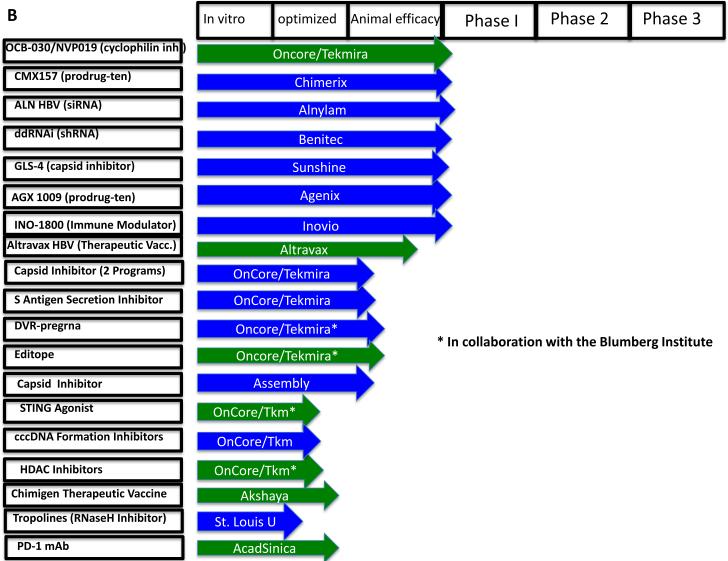
The most immediate new wave of therapies is likely to come from investigational agents currently in later stages of clinical development. As shown in Figures 4A & B, using publically available sources such as scholarly publications, the US government website www.clinicaltrials.gov, and individual pharmaceutical company websites, we found at least 38 new investigational agents under development for the management of chronic hepatitis B, of which 19 have reached human trials. Of the 38 investigational agents, we have designated 21 as DAAs and 17 as HTAs. The latter can be further sub-categorized as either immunomodulators (HTA-i) or targeting other host functions (HTA-hf) needed by the virus. Of the 19 drugs reported to have reached human trials, 3 have failed or been discontinued for commercial reasons. When we were unable to determine a drug's status, it is designated “status uncertain”.
Figure 4.
Therapeutics in development for the management of chronic HBV infection. (A) Investigational agents in Phase 1 clinical trials at the time of writing this review. The NCT number (wherever applicable) after the company's name is the clinicaltrials.gov identifier. (B) Investigational agents in preclinical stages. The stage of development is indicated, from in vitro identification through animal efficacy and ultimately human clinical trials. DAAs are highlighted in green and HTAs in blue. Investigational agents that have failed or have been stopped are shown in red. See text for citations. When we were unable to find a published reference, we cite the sponsor's website.
3. Direct-acting antivirals
3.1 New investigational agents now in clinical trials
3.1.1 Prodrugs of HBV polymerase inhibitors
Prodrugs are chemical or molecular precursors of active drugs (Hostetler, 2009; Smith, 2007). Typically, a prodrug is designed to improve the performance of the active drug substance, usually by decreasing toxicity, improving solubility, enhancing tissue absorption and/or increasing the half-life, so that the agent can be dosed no more frequently than once daily. Thus, as long as the prodrug can be efficiently and safely converted to the active agent, it may have better efficacy and safety profile. Prodrugs that reduce toxicity and improve pharmacokinetics are certainly welcome, although their contributions to care may be more incremental than transformational due to the persistence of cccDNA in the nucleus of infected hepatocytes and incomplete inhibition of HBV polymerase.
Prodrugs of tenofovir are in the most advanced stages of development (Figure 4A). As mentioned, earlier, the five approved polymerase inhibitors are currently used at doses that impressively reduce viremia, but have a much more modest impact on intracellular HBV DNA levels (Lau et al., 2005; Peters et al., 2004; Werle-Lapostolle et al., 2004). These drugs achieve effective suppression of viremia by 5-9 log10 or greater, but intrahepatic HBV viral DNA, although reduced, remains at significant levels. These reduced levels of replication are apparently sufficient to restore nuclear pools of cccDNA through intracellular recycling of nucleocapsids, even in people for who are serologically “PCR negative” (aviremic) for HBV.
That said, if a prodrug of a polymerase inhibitor could be safely used at higher doses or could achieve increased bioavailability of the active compound within hepatocytes, so as to achieve greater inhibition of the HBV polymerase, it could have a greater than incremental value. Indeed, since current polymerase inhibitors are associated with the loss of HBsAg and the appearance of HBsAb in as many as 10% of those treated over 5 years (Zoulim and Durantel, 2015), it is possible that a prodrug that inhibits intrahepatic DNA replication could significantly increase seroconversion. Several prodrugs at various clinical stages of development target the HBV polymerase.
AGX1009 and TAF
AGX1009 (Agenix) and TAF (Gilead), are prodrugs of tenofovir in Phase 3 clinical trials, although TAF is in Phase 3 for HIV indications and Phase 1/2 for hepatitis B (Menendez-Arias et al., 2014). They are predicted to have reduced long-term toxicity compared to Tonofovir.
Besifovir, elvucitabine, pradefovir mesylate & MIV210
Besifovir (LBO80380/ ANA380) 9-[1-(Phosphonomethoxycyclopropyl) methyl] guanine (PMCG) by Idong Pharma (Korea) is in Phase 3 clinical trial (Yuen et al., 2010). Achillion's elvucitabine is a L-cytosine nucleoside analog reverse transcriptase inhibitor that demonstrated potent antiviral activity against HBV and HIV. Phase 2 clinical studies showed that elvucitabine is well tolerated in patients with chronic HBV infection, with an antiviral potency is similar to that of lamivudine (Achillion Pharmaceuticals, 2010). No information is available regarding the efficacy of elvucitabine against lamivudine resistant HBV.
On the other hand, pradefovir mesylate, a propylated adefovir that depends upon CYP3A mediated activation, looked promising in Phase 2 studies, but was put on hold because of tumor formation in animals (Reddy et al., 2008). The development of MIV210 (Michalak et al., 2009) has also been abandoned (Grogan, 2013). Thus, even for prodrugs that have an established mechanism of action that is clearly beneficial, it is impossible to predict the “winners and losers” prior to preclinical and/or clinical evaluation.
CMX157
CMX157 (Chimerix/Contravir) is a lipid conjugate (hexadecycloxypropyl adenine) of tenofovir diphosphate that was designed to exploit lipid uptake pathways (Painter et al., 2007). CMX 157 delivers tenofovir diphosphate at high concentration in the hepatocytes, thus increasing the bioavailability of tenofovir diphosphate and at the same time decreasing circulating tenofovir levels to minimize potential renal side effects (Contravir). It has utility for both HIV and HBV, and is now entering Phase I/ 2 clinical trials for HBV.
3.1.2 siRNA
In principle, siRNA-acting drugs, which target HBV transcripts, should be able to shut down all HBV gene product production. This approach has had great promise, but has been frustrated by the inefficiency in delivery of the nucleic acid oligomers to human hepatocytes, despite extremely compelling results in experimental animals (Wooddell et al., 2013). Thus, if the delivery problem could be solved, the potential for siRNA and similar nucleic acid-directed suppressive molecules, is tremendous. It is with this hope and expectation, that a new wave of siRNA molecules is greeted (see forthcoming review by Gish and colleagues in this symposium).
ARC-520
ARC-520 is the siRNA from Arrowhead, which is lipid conjugated and uses nanoparticle-assisted delivery system. ARC-520 demonstrated good efficacy in reducing the levels of serum HBsAg, HBeAg and HBV DNA levels, in non-transgenic mouse model for HBV infection (Wooddell et al., 2013). It also showed promising results in HBV-infected chimpanzee model system. ARC-520 reached Phase 2 trials, but was placed on a clinical hold, and only recently it has been allowed to proceed with the Phase 2 studies (Arrowhead, 2015a).
ALN-HBV, TKM HBV
The ALN-HBV by Alnylam and TKM-HBV by Tekmira use lipid nanoparticle technology for delivering their siRNA. The Alnylam siRNA candidate demonstrated significant suppression of circulating HBV DNA and HBsAg levels in chimpanzee model system (Alnylam, 2014). The siRNA therapeutic candidates from these companies will have reached clinical phase by the time of this review's publication. The Tekmira siRNA agent is likely to be in at least Phase 1 clinical trial. Thus, despite recent reports of disappointing Phase 2 results for the Arrowhead compound (Arrowhead, 2015b; Wooddell et al., 2013) it still appears that, after the prodrugs, the siRNA technologies are the furthest along in development.
3.1.3 HBsAg-reducing agents
RepA9, from Replicor, is a nucleic acid-based polymer (NAP), comprised of phosphorothioated nucleic acids (Noordeen et al., 2013) (see forthcoming review by Vaillant and colleagues in this symposium). The sponsor reports that the agent is safe, and in small overseas human trials in Bangladesh, to have beneficial activity in combination with interferons or Zadaxin (Mahtab MA, 2015). The mechanism of action is unclear, but the sponsor reports it acts on HBsAg. As stated, compounds that act on HBsAg are particularly interesting because they also have the potential for direct activity against hepatitis delta virus (HDV), since HDV infection is dependent upon the HBsAg (Seeger and Mason, 2000).
3.1.4 Inhibitors of capsid formation
Of the DAAs that have reached clinical phase, at least three inhibit the formation of the HBV capsid. Since the first report of a capsid inhibitor more than 10 years ago (Stray et al., 2005; Stray and Zlotnick, 2006; Weber et al., 2002), there have been a number of new examples of this approach (see forthcoming review by Zlotnick et al. in this symposium). Capsid formation is an essential viral process that does not occur in the uninfected cell, and thus would be expected to provide a virus-selective target. Moreover, capsid proteins are readily detected in the nucleus of infected cells, far from the site of nucleocapsid formation in the cytoplasm. This is consistent with evidence that capsid proteins play a role in regulating HBV cccDNA expression and stability, as well as in regulating host innate immune response genes. Therefore, even though investigational agents may have a phenotypically similar effect on capsid assembly, they may modulate these other processes differently, thus affecting the overall ability of the agent to manage chronic HBV infection.
Three capsid inhibitors have reached clinical phase development: BAY4109 (AiCuris), NV1221 (Novira) and GLS 4 (Sunshine).
BAY4109
The capsid inhibitor BAY4109 came on the scene with a great deal of fanfare, as an innovative, first in class capsid inhibitor (Deres et al., 2003; Weber et al., 2002). It is reported to be highly species specific, for the virus, with activity against only the human HBV. This rendered pre-clinical efficacy testing more limited, since testing in woodchucks would not be possible, as it does not have activity against WHV. It was evaluated in Phase 1 clinical trials but its current development status is unclear.
NV1221
In the US, the Novira agent, NV1221, is probably the most advanced of the new capsid inhibitors, now in Phase 1 studies in New Zealand. There is no structural or specific functional information available about the compound, but it appears to prevent HBV capsid formation in a way analogous, but (importantly) not identical to the BAY4109.
GLS-4
GLS4 is a heteroaryl pyrimidine analogue that was derived from BAY4109 after structural optimization. GLS4 has a unique mechanism of action by which it causes aberrant capsid protein formation. GLS 4 was shown to inhibit nucleoside-analogue resistant HBV mutants in preclinical studies (Wang et al., 2012; Wu et al., 2013). HEC pharma group reports that Phase 1 studies of GLS4 are completed (HEC).
3.2 Direct-acting antivirals in preclinical development
3.2.1 Inhibitors of capsid morphogenesis
Preclinical phase capsid inhibitor candidates include CpAMs (Assembly Biosciences), DVR (Oncore-Tekmira), and DSS (Oncore-Tekmira). All these capsid inhibitors are small molecules that interfere with HBV capsid morphogenesis, but not necessarily at the same step. CpAMs are HBV core protein allosteric modulators that accelerate a dysfunctional capsid protein dimerization (Katen et al., 2013). DVRs prevent the association of HBV pregenomic RNA with the capsid (Campagna et al., 2013). The mechanism of action of the DSS compounds is not yet reported (Cai et al., 2012).
3.2.2 Inhibitors of HBsAg secretion
The development and current status of inhibitors of HBsAg secretion are reviewed by Cuconati and colleagues in a forthcoming article in this symposium. TTP is a small molecule that has been shown to prevent the secretion of HBsAg and viral DNA in vitro, possibly by interfering with the ability of HBsAg to associate with the LDL secretion machinery (Dougherty et al., 2007; Yu et al., 2011). HBsAg may also have immunosuppressive functions (Jaroszewicz et al., 2010; Xu et al., 2009). The TTPs are at an early preclinical stage of development, but are the only small molecule inhibitors of HBsAg secretion.
3.2.3 RNase H inhibitors
Unlike other DNA viruses HBV replication depends upon the RNAseH activity of HBV polymerase to degrade pregenomic RNA (Seeger and Mason, 2000). RNAseH enzymatic activity should, in principle, be a viable antiviral target as is the reverse transcriptase/DNA polymerase activity of HBV polymerase. A group in St Louis University (Cai et al., 2014; Tavis and Lomonosova, 2015) has reported identifying “hit” compounds, some based on those that are validated HIV drugs, that are selective inhibitors of the HBV polymerase RNAseH activity (see review by Tavis and Lomonosova in this symposium). These compounds need further development and could be a welcome addition to the HBV antiviral arsenal. They can prove be very effective when used in combination with the existing nucleot(s)ide analogues and may help to achieve long-term inhibition of HBV replication at a level that is not achieved by current nucleot(s)ide analogues alone.
3.2.4 CRISPR/Cas9 system
The bacterial clustered regularly interspaced short palindromic repeats associated systems (CRISP/Cas9) loci encode RNA guided endonucleases, derived from bacterial immune response against foreign genetic elements such as bacteriophages (Kennedy et al., 2015; Seeger and Sohn, 2014) and have been adapted for mammalian systems (see forthcoming review by Cullen and colleagues in this symposium). In principle, they can be used to target destruction of specific DNA sequences, and thus hold a great potential for specific degradation of HBV cccDNA. The challenges of getting these complex systems into hepatocytes, let alone into the nucleus, are clear. However, lentiviruses expressing CRISPR/Cas9 guide RNAs that are specific for HBV DNA have been transduced into HBV cccDNA-producing cells and shown to be suppressive (Dong et al., 2015; Kennedy et al., 2015; Lin et al., 2014). HepG2 cells expressing the receptor were infected with HBV and the CRISPER/Cas9 system was used to induce degradation of cccDNA. This also suggested that the targeted DNA is degraded rather than repaired following Cas9 nuclease digestion. Thus, there is some progress with these systems, although clinical investigation is probably a long way off due to the difficulties in the delivery process.
3.2.5 siRNA
ddRNAi from Benitech is another therapeutic approach to directly target HBV transcripts using RNA interference technology. This program was initiated by Benitech in 2009 and is currently in preclinical stages. The studies are being conducted in collaboration with Chinese-based “Biomics biotechnologies”, and are aimed at targeting HBV polymerase transcript using three different short-hairpin RNA (shRNA) to target different regions of the polymerase transcript (Biopharma).
4. Host-targeting antivirals
4.1 Products in clinical development
Five HTAs appear to be in the clinical stage of development (Fig. 4), and two others have recently failed or been discontinued. Of the five still in development, two target host functions used by the virus (Myrcludex B, Brinapant), and the others target the host innate and adaptive immune systems (GS-4774, GS 9620, DV601 & SB9200HBV).
4.1.1 Viral entry inhibitors
Myrcludex B (MycB), a synthetic lipopeptide derived from the LHBs preS1 domain, is an entry inhibitor from Hepatera/Myr-GmbH. Viral entry inhibitors are a relatively new and effective antiviral drugs, with the HIV Trimeris Fuzeon being among the first (Lobritz et al., 2010). Neutralization of virions to prevent their association with target cells by neutralizing antibodies is well established as an approach. For HBV, the only example is Myrcludex B (MycB), a lipopeptide derived from the cell attachment region of the HBV large envelope protein (Gripon et al., 2005; Petersen et al., 2008; Urban et al., 2014) Myrcludex B requires parenteral administration; this would be a drawback for clinical therapy. In Phase 2a clinical trial studies Myrcludex B demonstrated does dependent decline in the levels of HBV DNA in patients. Myrcludex B also showed promising results in phase 2a trial in patients with chronic hepatitis delta virus (HDV) infection. HDV uses HBV envelope proteins for hepatocyte entry and therefore if Myrcludex B is approved it can be used for treating both HBV and HDV chronic infections. (Hepatera, 2014; Urban et al., 2014).
4.1.2 Immune enhancers
Four of the HTAs in clinical phase are immune enhancers. The mechanism of liver disease in people with chronic hepatitis B is fundamentally a chronic, but inadequate immunological and inflammatory attack on infected hepatocytes, resulting in liver damage (Ganem and Prince, 2004; Seeger and Mason, 2000). A DAA alone may therefore not be sufficient; a durable, off-drug, antiviral response is therefore likely to require some type of immunological restoration of the affected individual. While it is hoped that complete suppression of antigenemia, as well as viremia, may unmask or enable an indolent or suppressed immune response (or perhaps “free up” neutralizing antibody that was otherwise complexed with antigen), some type of direct immunologic awakening of the host will be necessary (see review by Durantel and colleagues in this symposium).
Unfortunately, most attempts to stimulate the immune system in chronic HBV carriers to clear HBV have been disappointing, and have failed in human trials or had untoward clinical consequences, such as decompensation in those with advanced fibrosis or cirrhosis. Therefore there has been considerable interest in developing strategies that stimulate the host immune recognition of HBV, in chronic carriers, who usually can recognize the virus, at the cellular and humoral level, without clearing the infection (Bertoletti and Gehring, 2013).
YIC
YIC is comprised of HBsAg and HBIG, (anti-HBs Immunoglobulin) complex as a therapeutic agent candidate with alum as the adjuvant (Xu et al., 2008). The candidate reached phase III clinical trials in which the results were found to be unsatisfactory. Overstimulation with YIC was demonstrated to decrease the efficacy of YIC due to immune fatigue (Xu et al., 2013). Its continuation is therefore in doubt, representing a setback and disappointment for these alternative approaches once thought promising.
GS 9620
Toll-like receptor (TLR)-7 is a “pathogen recognition receptor” expressed mostly in lysosomal/endosomal compartments of plasmacytoid dendritic cells (pDCs) and B-lymphocytes that recognizes patterns in viral single-stranded RNA (O'Neill et al., 2013). There is a growing body of evidence that pattern recognition receptor activation can directly suppress HBV in infected cells (Chang et al., 2012) (see forthcoming review by Chang and colleagues in this symposium).
GS 9620 is a small-molecule TLR-7 agonist in clinical development (Roethle et al., 2013). Phase 1b safety studies have been recently reported, showing the drug was safe and achieved expected induction of interferon stimulated genes in peripheral blood cells (Gane et al., 2015). Indeed, GS9620 had significant activity in woodchucks and chimps, and would be a “first in class”, to show that pharmacological activation of pattern recognition receptor can have clinical benefit in the management of chronic HBV infection (Lanford et al., 2013).
SB 9200
HBV replication is suppressed by activation of retinoic acid inducible gene-I (RIG I), which is stimulated by double-stranded RNA and the nucleotide binding oligomerization domain containing protein-2 (NOD-2) (Adam et al., 2002; Mao et al., 2011; Sato et al., 2015). Agents that can activate RIG-I would therefore provide a new approach. SB 9200 from Spring Bank is reported on their web site to be a small molecule that activates host's immune system by upregulating RIG I SB 9200 is currently in Phase 1 clinical trials (Springbank).
4.1.3. Therapeutic vaccines
The concept and current status of therapeutic vaccination for chronic hepatitis B are reviewed in a forthcoming article by Roggendorf and colleagues in this symposium.
DV601 (Dynavax) and GS4774 (Gilead Sciences) are both therapeutic vaccines candidates. GS4774 is a heat-killed vaccine engineered to express a fusion protein containing HBsAg sequences of four major genotypes while DV601 utilizes both core and surface antigens. Vaccine that produce or introduces HBsAg might appear to have limited logic, since people with chronic hepatitis B already have enormous amounts of HBsAg in their circulation. The hypothesis behind this approach is that therapeutic vaccines will induce specific T-cell responses, in the face of presumed T-cell exhaustion due to antigen excess, by either stimulating antigen presentation, or directing antigen presentation from professional antigen presenting cells (Michel et al., 2015). Some of these challenges are discussed later in this review. As with any immune-enhancing approach, the limitations will most likely relate to toxicity and variable response in different patients.
To date, GS4774, produced from heat-inactivated yeast recombinant HBV antigens, has been evaluated in Phase 1 trials (Gaggar et al., 2014). The Dynavax therapeutic vaccine candidate, which includes phosphorothioate oligonucleotides as well as viral polypeptides, as immunostimulatory adjuvants, has also been shown to be safe and effective as an immunogen in Phase 1 studies (Halperin et al., 2006; Plotkin and Schaffner, 2013). Since the initial safety trials of DV601, no development has been reported for the vaccine candidate (DYNAVAX, 2011).
4.1.4 Birinapant
Birinapant from Tetralogic Corp, is a small molecule that is believed to mimic second mitochondrial activator of caspases (SMAC) (Seigal et al., 2015). SMAC normally binds to IAP (inhibitor of apoptosis), pushing the cell towards apoptosis (Holohan et al., 2013).
Birinapant has already entered Phase 3 trials for management of myelodysplastic syndrome and colorectal cancer, so there is considerable safety and efficacy information available. The safety information on Birinapant is available from more than 300 patients enrolled in these trials (TetraLogic). For the management of hepatitis B, Birinapant is only in the preclinical phase, but was reported to cause impressive reductions of viremia in the circulation of infected mice (Ebert et al., 2015a; Ebert et al., 2015b; Peters et al., 2004). The mechanism of the antiviral affect was not clear, but it seems that infected cells are selectively eliminated, assuming that they are more sensitive to apoptotic stimuli, compared to the uninfected cells. The strategy is intriguing and the possibility of using such an approach to eliminate remaining infected cell “nests” following the reduction of infected cells with more conventional methods, is very compelling. On the other hand, for obvious reasons, in individuals with a substantial portion of the liver infected, such an approach must be taken cautiously, since the rapid destruction of infected cells can be dangerous.
4.1.5 Zadaxin
Zadaxin is a 28-amino-acid peptide based on thymosin-α, a natural polypeptide from the thymus (Tsai et al., 2003). It was reported to initially show promise, but there are studies demonstrating both favorable and unfavorable results (Wu et al., 2015). A randomized clinical trial conducted in China demonstrated that Zadaxin is safe, well tolerated and is effective in inhibiting HBV replication. Patients treated with Zadaxin had much higher rate of seroconversion as compared to the patients treated with IFN-α (You et al., 2001). In another clinical trial, HBeAg negative patients treated with Zadaxin in combination with IFN-α had much robust virological and biochemical response as compared to the patients treated with combination of lamivudine and IFN-α or IFN-α alone (Saruc et al., 2003). In contrast, Zadaxin in combination with lamivudine was not found to be any better than lamivudine alone (Lee et al., 2008). Thymosin-α also did not have any clear benefits when used in combination with IFN-α in another clinical trial (Yang et al., 2008). Subsequent studies showed that, in combination with lamivudine or interferon, Zadaxin added little to detectable benefit in chronically infected HBeAg-positive patients, on the grounds of antiviral efficacy, and interest has waned (Kim et al., 2012).
4.2 HTAs in the preclinical stage
4.2.1 Immune checkpoint inhibitors (PD-1)
Recent advancements in our understanding of immune exhaustion is generating a new hope for the immunological or host-targeted therapeutic strategies. We now have a greater understanding of the mechanisms by which some viral antigens, in chronic infection, are presented to the immune system that can result in the failure of the immune system to clear the virus. There are now several known pathways that lead to T-cell exhaustion and these pathways could be blocked to enhance T-cell responses.
For example, the programmed cell death (PD-1)/ PD-1 ligand pathway plays critical role in antigen-mediated exhaustion of T-cells in several chronic infections, including HIV and hepatitis C (Bertoletti and Gehring, 2007; Bertoletti and Gehring, 2013; Bertoletti and Kennedy, 2014; Day et al., 2006; Peng et al., 2008). In the hydrodynamic tail vein injection model system for HBV expression in C57BL mice (Tzeng et al., 2012), it was demonstrated that a PD-1/PD-L1 pathway inhibition with monoclonal antibody could reverse immune dysfunction and HBV viral persistence. PD-1 expression levels in HBV-infected patients have also been studied, and it was demonstrated that 70% of the circulating HBV-specific T-cells were PD-1 positive (Zhang et al., 2013).
Anti-PD-1 antibody has now been used effectively in human cancer therapy, and in trials for the management of chronic hepatitis C (Gardiner et al., 2013). However, there is no ongoing clinical trial involving PD-1 for the management of chronic HBV infection. This may be due to concerns about adverse effects of autoimmune type reactions. Thus, despite the theoretical appeal, and strong experimental evidence, PD-1-derived therapies for chronic hepatitis B may take some time to develop. However, other members of the PD-1-like superfamily of coreceptors may be important in regulating chronicity, and ultimately prove better therapeutic targets (Xing and Hogquist, 2012).
4.2.2 Therapeutic vaccines
Therapeutic vaccines have received a considerable amount of attention, and the clinical stage therapeutic vaccines for HBV have been discussed (above). Here we describe leading examples of preclinical technologies and their development status, when it could be confirmed.
Altravax DNA vaccine
Altravax's therapeutic vaccine approach is to construct a chimera of wild-type and xenogenic HBs surface peptides, and subsequent inclusion of HBc peptides. This approach is based upon the hypothesis that the chimera will result in the presentation of novel epitopes by antigen presenting cells. These novel epitopes will beneficially stimulate the immune response in chronic HBV infection. C57BL/6 mice were immunized with the vaccine, and the T-cell responses elicited by the vaccine were reported to meet the expectations by the investigators (Altravax).
INO-1800
INO-1800 is a recombinant DNA vaccine from Inovio that encodes consensus sequence of HBV core antigen, which has induced antigen-specific strong T-cell responses and high antibody titers in preclinical trials (Obeng-Adjei et al., 2012). The vaccine also demonstrated strong cytotoxic T-cell response to kill target cells without causing considerable liver injury (Obeng-Adjei et al., 2012). The company has just initiated Phase1 trials for the vaccine (Inovio, 2015)
VLP
VLP Biotech is developing a virus-like particle (VLP) (Schickli et al., 2015), based on the HBV core antigen, modified and designed to elicit neutralizing antibodies to PreS1 (Whitacre et al., 2009).
Chimigen HBV/NU500
This investigational agent from Akshaya Bio (Canada) is a chimeric polypeptide comprised of regions of the core and surface antigen proteins and the Fc-binding domain of IgG. The hypothesis is that the Fc component will direct the chimeric protein to dendritic antigen presenting cells, and there will be enhanced presentation of HBV antigen. The concept is compelling, and in vitro and in vivo studies reported at professional conferences, suggest that this approach can induce robust immunological responses to the HBV antigens, in mice (George, 2014). However, successful production and presentation of viral antigens may be insufficient to restore a meaningful host immunological response to HBV, since there may be an “afferent” defect in the immunologic response in all individuals with chronic hepatitis B, known as “immunological exhaustion”. As mentioned earlier, immune exhaustion due to the pathways such as PD-1/PD-1L might undermine the effects of enhanced antigen presentation.
Editopes
A novel method by which a small molecule can induce production of an HBsAg peptide bearing a novel epitope has been described (Norton et al., 2010). Briefly, imino sugar glucosidase inhibitors such as N-butyldeoxynojirimycin, which inhibit alpha-glucosidase 1, prevent the glycan processing of HBsAg (M) polypeptides, causing them to misfold and be degraded in the proteasomes (Norton et al., 2010; Simsek et al., 2009). Prior to their degradation, the N-glycans on the HBsAg are removed from the polypeptide backbone by the action of PNgase, which hydrolyzes the asparagine linked to the N-glycan, resulting in its conversion into an aspartic acid. The proteolytic fragment of the HBsAg thus contains a CTL epitope that is not encoded by the HBV gene. Since this amino acid resides within a major CTL epitope, the new epitope, called an editope, contains an aspartic acid in place of an asparagine.
This suggests that animals (and presumably, humans) could be treated with the imino sugar, and immunized with the novel CTL epitope (editopes), and, ideally, would develop CTL responses that would attack the infected cells. The hypothesis was evaluated in woodchucks chronically infected with woodchuck hepatitis virus (WHV), and it was demonstrated that selective lymphocytic responses to the aspartic acid containing editope could be induced in a pharmacologically dependent manner (Norton et al., 2010). However, there was no detectable effect on viremia or antigenemia. Thus, despite an immunological proof of principle, this approach has not progressed further.
4.2.3 Epigenetic modifiers
Small-molecule histone deacetylase (HDAC) inhibitors have been shown to suppress cccDNA transcription in tissue culture, under non-cytotoxic conditions (Liu et al., 2013). At the same time, transcription from HBV DNA integrated into the host genome was enhanced, demonstrating that transcription from cccDNA is regulated differently than the transcription from the integrated genome. These results are particularly compelling, since there are several HDAC inhibitors in Phase 3 clinical studies for other diseases, and two are already approved for refractory cutaneous and peripheral T cell lymphoma (Khan and La Thangue, 2012; West and Johnstone, 2014). The positive results of the use of HDAC inhibitors for suppressing cccDNA is therefore exciting, since two of them have already been approved for other indications, but of course, as with any host targeting agent, human use may pose risks.
4.2.4 Dimethylxanthenone STING agonists
The Stimulator of Interferon Genes (STING) is an adaptor polypeptide for several cytoplasmic DNA-sensing receptors, as well as a bacterial cyclic di-nucleotide second messenger and an intracellular moderator of innate immune responses (Cai et al., 2014). Stimulation of STING with small-molecule flavonoids effectively suppressed HBV replication in mouse hepatocytes. It was demonstrated that STING stimulation mainly induced type-1 IFN response, unlike the TLR agonist that induced inflammatory cytokines response (Guo et al., 2015). Importantly, it has been demonstrated that after less than 24 hours of infection, STING agonists reduced the amount of HBV DNA in the blood of infected mice in a hydrodynamic tail-vein injection model (Guo et al., 2015).
4.2.5 Cyclophilin inhibitors
Cyclophilin inhibitors have been shown to have broad antiviral affects in clinical trials for hepatitis C (Membreno et al., 2013). The mechanism of action appears to involve an affect upon host protein-folding chaperones used in viral polypeptide function.
The cyclophilin inhibitors Alisporivir (formerly Debio 025) and Novartis compound NIM811 have both been reported to have inhibitory activity against HBV in tissue culture (Phillips et al., 2015). Oncore-Tekmira is now developing a cyclophilin inhibitor (OCB-030/NVPO19) for the management of chronic hepatitis B. Cyclosporin A, a chemical inhibitor of cyclophilin that binds and inhibits cyclophilin without any immunosuppressive effects, may also be important for inhibiting entry in hepatocytes (Nkongolo et al., 2014).
5. Conclusions
It has been 50 years since the discovery of the Australia antigen, but therapies that cure chronic HBV infection are still not available. In contrast, 25 years after the discovery of the hepatitis C virus, virtually all cases of chronic hepatitis C can now be cured through a short course of antiviral therapy (Ward, 2014). This dramatic success with hepatitis C has created excitement about the possibility of a cure for chronic hepatitis B, and many scientists are now moving to hepatitis B research. Coupled with the growing prominence of antiviral research in China and the rest of South Asia, where hepatitis B is prevalent (El-Serag, 2012), this has created a momentum of innovation in hepatitis B therapeutic strategies.
As discussed in this review, investigational agents currently in the advanced stages of clinical development are the prodrugs that have promise to improve upon the existing therapies by increasing efficacy and decreasing the side-effects. Investigational agents such as siRNA, HBsAg inhibitors and capsid inhibitors that have reached clinical trials, have different mechanism of action as compared to the current therapies. The new delivery technologies such as lipid nanoparticles have breathed new life in to the siRNA approach. New technologies for discovery, screening and profiling, have also made possible the development of many investigational agents in the pre-clinical phase of development that use novel and previously unexplored areas of HBV biology. These include entry inhibitors, use of novel chimeric epitopes, selective killing of infected hepatocytes and novel therapeutic vaccines. There is a hope that these investigational agents that use novel mechanisms for inhibiting HBV replication, used alone or in combination with the existing therapies would help in better management of chronic hepatitis. The great challenge is to decrease intrahepatic viral DNA levels and to eliminate cccDNA. Candidate therapies such as siRNA hold promise to do that by eliminating HBV transcripts, eventually leading to loss of cccDNA, over a period of time, and therefore the results from the clinical trials are awaited. In addition to the DAAs, it is thought that agents that can boost the immune response against the virus can play an important role in sustained off drug virological responses. Therefore new HTAs in development, such as therapeutic vaccines, PD-1/PD-1ligand pathway inhibitors, novel chimeric antigens, enhancement of antigen presentation, might be beneficial.
That said, to date there are still only two families of drugs to treat chronic hepatitis B, the interferons and the polymerase inhibitors. But this is clearly going to change. Based on some of the investigational agents in the pipeline that we describe in this review, we predict that within five years there will be at least two new drugs approved for management of chronic hepatitis B, and within ten years, there should be functional cures.
Highlights.
This articles introduces a symposium marking the 50th anniversary of the discovery of the Australia antigen.
Current antiviral therapies for chronic hepatitis B suppress viral replication, but rarely eradicate the virus.
The persistence of viral cccDNA in the nucleus of hepatocytes is the principal obstacle to curative therapy.
Many new treatment approaches are now under development, and some may be more effective in eliminating cccDNA.
As for hepatitis C, optimal therapy for hepatitis B may consist of a combination of drugs with different targets.
Acknowledgements
The preparation of this manuscript was supported in part by funding from NIH grant RO1 AI104636, by the Baruch S. Blumberg Institute and by the Commonwealth of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam BL, Qu Y, Davis JW, Ward MD, Clements MA, Cazares LH, Semmes OJ, Schellhammer PF, Yasui Y, Feng Z, Wright GL., Jr. Serum protein fingerprinting coupled with a pattern-matching algorithm distinguishes prostate cancer from benign prostate hyperplasia and healthy men. Cancer research. 2002;62:3609–3614. [PubMed] [Google Scholar]
- Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. The New England journal of medicine. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, Petersen J, van Bommel F, de Knegt RJ, Santantonio T, Berg T, Welzel TM, Wedemeyer H, Buti M, Pradat P, Zoulim F, Hansen B, Janssen HL. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2014 doi: 10.1136/gutjnl-2014-307023. [DOI] [PubMed] [Google Scholar]
- Arrowhead Arrowhead Provides Update on IND for ARC-520 Phase 2b Study. 2015b.
- Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531–540. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- Bedossa P. Reversibility of hepatitis B virus cirrhosis after therapy: who and why? Liver international : official journal of the International Association for the Study of the Liver. 2015;35(Suppl 1):78–81. doi: 10.1111/liv.12710. [DOI] [PubMed] [Google Scholar]
- Bertoletti A, Gehring A. Immune response and tolerance during chronic hepatitis B virus infection. Hepatology research : the official journal of the Japan Society of Hepatology. 2007;37(Suppl 3):S331–338. doi: 10.1111/j.1872-034X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- Bertoletti A, Gehring AJ. Immune therapeutic strategies in chronic hepatitis B virus infection: virus or inflammation control? PLoS pathogens. 2013;9:e1003784. doi: 10.1371/journal.ppat.1003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cellular & molecular immunology. 2014 doi: 10.1038/cmi.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Gish R, Guo H, Mehta A, Cuconati A, Thomas London W, Guo JT. Chronic hepatitis B: What should be the goal for new therapies? Antiviral research. 2013;98:27–34. doi: 10.1016/j.antiviral.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11:685–706. vii. doi: 10.1016/j.cld.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093–5107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- Blumberg BS, Alter HJ, Visnich S. A “New” Antigen in Leukemia Sera. JAMA : the journal of the American Medical Association. 1965;191:541–546. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, Streinu-Cercel A, Wang JY, Idilman R, Reesink HW, Diculescu M, Simon K, Voiculescu M, Akdogan M, Mazur W, Reijnders JG, Verhey E, Hansen BE, Janssen HL. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology. 2015;61:1512–1522. doi: 10.1002/hep.27586. [DOI] [PubMed] [Google Scholar]
- Cai CW, Lomonosova E, Moran EA, Cheng X, Patel KB, Bailly F, Cotelle P, Meyers MJ, Tavis JE. Hepatitis B virus replication is blocked by a 2-hydroxyisoquinoline-1,3(2H,4H)-dione (HID) inhibitor of the viral ribonuclease H activity. Antiviral research. 2014;108:48–55. doi: 10.1016/j.antiviral.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM, Cuconati A, Guo H. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrobial agents and chemotherapy. 2012;56:4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna MR, Liu F, Mao R, Mills C, Cai D, Guo F, Zhao X, Ye H, Cuconati A, Guo H, Chang J, Xu X, Block TM, Guo JT. Sulfamoylbenzamide derivatives inhibit the assembly of hepatitis B virus nucleocapsids. Journal of virology. 2013;87:6931–6942. doi: 10.1128/JVI.00582-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HB, Hann HW. Time for an active antiviral therapy for hepatitis B: An update on the management of hepatitis B virus infection. Therapeutics and clinical risk management. 2007;3:605–612. [PMC free article] [PubMed] [Google Scholar]
- Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, Kao JH, Jia JD, Wedemeyer H, Locarnini S, Janssen HL, Marcellin P. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. Journal of hepatology. 2011;55:1121–1131. doi: 10.1016/j.jhep.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Chang J, Block TM, Guo JT. The innate immune response to hepatitis B virus infection: implications for pathogenesis and therapy. Antiviral research. 2012;96:405–413. doi: 10.1016/j.antiviral.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, Cross A, DeHertogh D, Wilber R, Colonno R, Apelian D. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. The New England journal of medicine. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014 doi: 10.1136/gutjnl-2013-306409. [DOI] [PubMed] [Google Scholar]
- Curry MP, Forns X, Chung RT, Terrault NA, Brown R, Jr., Fenkel JM, Gordon F, O'Leary J, Kuo A, Schiano T, Everson G, Schiff E, Befeler A, Gane E, Saab S, McHutchison JG, Subramanian GM, Symonds WT, Denning J, McNair L, Arterburn S, Svarovskaia E, Moonka D, Afdhal N. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100–107. e101. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Deres K, Schroder CH, Paessens A, Goldmann S, Hacker HJ, Weber O, Kramer T, Niewohner U, Pleiss U, Stoltefuss J, Graef E, Koletzki D, Masantschek RN, Reimann A, Jaeger R, Gross R, Beckermann B, Schlemmer KH, Haebich D, Rubsamen-Waigmann H. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299:893–896. doi: 10.1126/science.1077215. [DOI] [PubMed] [Google Scholar]
- Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral research. 2015;118:110–117. doi: 10.1016/j.antiviral.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Dougherty AM, Guo H, Westby G, Liu Y, Simsek E, Guo JT, Mehta A, Norton P, Gu B, Block T, Cuconati A. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrobial agents and chemotherapy. 2007;51:4427–4437. doi: 10.1128/AAC.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASL EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of hepatology. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Ebert G, Allison C, Preston S, Cooney J, Toe JG, Stutz MD, Ojaimi S, Baschuk N, Nachbur U, Torresi J, Silke J, Begley CG, Pellegrini M. Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2015a doi: 10.1073/pnas.1502400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert G, Preston S, Allison C, Cooney J, Toe JG, Stutz MD, Ojaimi S, Scott HW, Baschuk N, Nachbur U, Torresi J, Chin R, Colledge D, Li X, Warner N, Revill P, Bowden S, Silke J, Begley CG, Pellegrini M. Cellular inhibitor of apoptosis proteins prevent clearance of hepatitis B virus. Proceedings of the National Academy of Sciences of the United States of America. 2015b doi: 10.1073/pnas.1502390112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. e1261. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, Subramanian GM, McHutchison JG. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32:4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye Z, Pflanz S, Garrison KL, Gaggar A, Mani Subramanian G, McHutchison JG, Kottilil S, Freilich B, Coffin CS, Cheng W, Kim YJ. The Oral Toll-Like Receptor-7 Agonist GS-9620 in Patients with Chronic Hepatitis B Virus Infection. Journal of hepatology. 2015 doi: 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. The New England journal of medicine. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PloS one. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. Chimigen® HBV therapeutic vaccine: A novel dendritic cell receptor-targeted immunotherapy for treating chronic Hepatitis B virus infection, $th World Congress on Virology; HIlton San Antonio Airport, TX, USA. 2014. [Google Scholar]
- Gish RG, Marrero JA, Benson AB. A multidisciplinary approach to the management of hepatocellular carcinoma. Gastroenterology & hepatology. 2010;6:1–16. [PMC free article] [PubMed] [Google Scholar]
- Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Vijayadeva V, Boscarino JA, Henkle EM, Oja-Tebbe N, Lu M. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. Journal of virology. 2005;79:1613–1622. doi: 10.1128/JVI.79.3.1613-1622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan K. Medivir and Daewoong pull plug on hepatitis B drug. PharmaTimes Digital. 2013 [Google Scholar]
- Guo F, Han Y, Zhao X, Wang J, Liu F, Xu C, Wei L, Jiang JD, Block TM, Guo JT, Chang J. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrobial agents and chemotherapy. 2015;59:1273–1281. doi: 10.1128/AAC.04321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, Levitt D, Nest GV, Gennevois D, Eiden JJ. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine. 2006;24:20–26. doi: 10.1016/j.vaccine.2005.08.095. [DOI] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature reviews. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Sherker AH. Therapy for hepatitis C--the costs of success. The New England journal of medicine. 2014;370:1552–1553. doi: 10.1056/NEJMe1401508. [DOI] [PubMed] [Google Scholar]
- Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral research. 2009;82:A84–98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, Cornberg M. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. Journal of hepatology. 2010;52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Kanwal F, El-Serag HB, Ross D. Surveillance for hepatocellular carcinoma: can we focus on the mission? Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13:805–807. doi: 10.1016/j.cgh.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Katen SP, Tan Z, Chirapu SR, Finn MG, Zlotnick A. Assembly-directed antivirals differentially bind quasiequivalent pockets to modify hepatitis B virus capsid tertiary and quaternary structure. Structure. 2013;21:1406–1416. doi: 10.1016/j.str.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EM, Bassit LC, Mueller H, Kornepati AV, Bogerd HP, Nie T, Chatterjee P, Javanbakht H, Schinazi RF, Cullen BR. Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology. 2015;476:196–205. doi: 10.1016/j.virol.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- Kim BH, Lee YJ, Kim W, Yoon JH, Jung EU, Park SJ, Kim YJ, Lee HS. Efficacy of thymosin alpha-1 plus peginterferon alpha-2a combination therapy compared with peginterferon alpha-2a monotherapy in HBeAg-positive chronic hepatitis B: a prospective, multicenter, randomized, open-label study. Scandinavian journal of gastroenterology. 2012;47:1048–1055. doi: 10.3109/00365521.2012.694902. [DOI] [PubMed] [Google Scholar]
- Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M, Rustgi V, Chojkier M, Herring R, Di Bisceglie AM, Pockros PJ, Subramanian GM, An D, Svarovskaia E, Hyland RH, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Pound D, Fried MW. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. The New England journal of medicine. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508–1517. 1517, e1501–1510. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. The New England journal of medicine. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- Lee HW, Lee JI, Um SH, Ahn SH, Chang HY, Park YK, Hong SP, Moon YM, Han KH. Combination therapy of thymosin alpha-1 and lamivudine for HBeAg positive chronic hepatitis B: A prospective randomized, comparative pilot study. Journal of gastroenterology and hepatology. 2008;23:729–735. doi: 10.1111/j.1440-1746.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- Lee YB, Lee JH, Choi WM, Cho YY, Yoo JJ, Lee M, Lee DH, Cho Y, Yu SJ, Kim YJ, Yoon JH, Kim CY, Lee HS. Efficacy of adefovir-based combination therapy for patients with Lamivudine- and entecavir-resistant chronic hepatitis B virus infection. Antimicrobial agents and chemotherapy. 2013;57:6325–6332. doi: 10.1128/AAC.01742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw L, Crawford HC. Functions of the extracellular matrix and matrix degrading proteases during tumor progression. Braz J Med Biol Res. 1999;32:805–812. doi: 10.1590/S0100-879X1999000700002. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, Amarapurkar D, Cooksley G, Jafri W, Mohamed R, Hou JL, Chuang WL, Lesmana LA, Sollano JD, Suh DJ, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatology International. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. The New England journal of medicine. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- Lin SR, Yang HC, Kuo YT, Liu CJ, Yang TY, Sung KC, Lin YY, Wang HY, Wang CC, Shen YC, Wu FY, Kao JH, Chen DS, Chen PJ. The CRISPR/Cas9 System Facilitates Clearance of the Intrahepatic HBV Templates In Vivo. Molecular therapy. Nucleic acids. 2014;3:e186. doi: 10.1038/mtna.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Campagna M, Qi Y, Zhao X, Guo F, Xu C, Li S, Li W, Block TM, Chang J, Guo JT. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS pathogens. 2013;9:e1003613. doi: 10.1371/journal.ppat.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobritz MA, Ratcliff AN, Arts EJ. HIV-1 Entry, Inhibitors, and Resistance. Viruses. 2010;2:1069–1105. doi: 10.3390/v2051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locarnini S. Molecular virology of hepatitis B virus. 2004. [DOI] [PubMed]
- Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303–309. doi: 10.1053/j.gastro.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Lok AS. Does antiviral therapy for hepatitis B and C prevent hepatocellular carcinoma? Journal of gastroenterology and hepatology. 2011;26:221–227. doi: 10.1111/j.1440-1746.2010.06576.x. [DOI] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- Lok AS, Ward JW, Perrillo RP, McMahon BJ, Liang TJ. Reactivation of hepatitis B during immunosuppressive therapy: potentially fatal yet preventable. Ann Intern Med. 2012;156:743–745. doi: 10.1059/0003-4819-156-10-201205150-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtab MA, B.M., Vaillant A. Effects of nucleic acid polymer therapy alone or in combination with immunotherapy on the establishment of SVR in patients with chronic HBV infection. 2015.
- Mao R, Zhang J, Jiang D, Cai D, Levy JM, Cuconati A, Block TM, Guo JT, Guo H. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis B virus in human hepatocyte-derived cells. Journal of virology. 2011;85:1048–1057. doi: 10.1128/JVI.01998-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Membreno FE, Espinales JC, Lawitz EJ. Cyclophilin inhibitors for hepatitis C therapy. Clin Liver Dis. 2013;17:129–139. doi: 10.1016/j.cld.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Menendez-Arias L, Alvarez M, Pacheco B. Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Current opinion in virology. 2014;8:1–9. doi: 10.1016/j.coviro.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Michalak TI, Zhang H, Churchill ND, Larsson T, Johansson NG, Oberg B. Profound antiviral effect of oral administration of MIV-210 on chronic hepadnaviral infection in a woodchuck model of hepatitis B. Antimicrobial agents and chemotherapy. 2009;53:3803–3814. doi: 10.1128/AAC.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel ML, Bourgine M, Fontaine H, Pol S. Therapeutic vaccines in treating chronic hepatitis B: the end of the beginning or the beginning of the end? Medical microbiology and immunology. 2015;204:121–129. doi: 10.1007/s00430-014-0381-y. [DOI] [PubMed] [Google Scholar]
- Nkongolo S, Ni Y, Lempp FA, Kaufman C, Lindner T, Esser-Nobis K, Lohmann V, Mier W, Mehrle S, Urban S. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. Journal of hepatology. 2014;60:723–731. doi: 10.1016/j.jhep.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrobial agents and chemotherapy. 2013;57:5291–5298. doi: 10.1128/AAC.01003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton PA, Menne S, Sinnathamby G, Betesh L, Cote PJ, Philip R, Mehta AS, Tennant BC, Block TM. Glucosidase inhibition enhances presentation of de-N-glycosylated hepatitis B virus epitopes by major histocompatibility complex class I in vitro and in woodchucks. Hepatology. 2010;52:1242–1250. doi: 10.1002/hep.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill AK, Niederst MJ, Newton AC. Suppression of survival signalling pathways by the phosphatase PHLPP. The FEBS journal. 2013;280:572–583. doi: 10.1111/j.1742-4658.2012.08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng-Adjei N, Choo DK, Saini J, Yan J, Pankhong P, Parikh A, Chu JS, Weiner DB. Synthetic DNA immunogen encoding hepatitis B core antigen drives immune response in liver. Cancer Gene Ther. 2012;19:779–787. doi: 10.1038/cgt.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter GR, Almond MR, Trost LC, Lampert BM, Neyts J, De Clercq E, Korba BE, Aldern KA, Beadle JR, Hostetler KY. Evaluation of hexadecyloxypropyl-9-R-[2-(Phosphonomethoxy)propyl]- adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrobial agents and chemotherapy. 2007;51:3505–3509. doi: 10.1128/AAC.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Pellicelli AM, Barbaro G, Francavilla R, Romano M, Barbarini G, Mazzoni E, Mecenate F, Paffetti A, Barlattani A, Struglia C, Villani R, Nauri L, Nosotti L, Armignacco O, Ferri F, Camporiondo MP, Soccorsi F. Adefovir and lamivudine in combination compared with adefovir monotherapy in HBeAg-negative adults with chronic hepatitis B virus infection and clinical or virologic resistance to lamivudine: a retrospective, multicenter, nonrandomized, open-label study. Clinical therapeutics. 2008;30:317–323. doi: 10.1016/j.clinthera.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Peters MG, Hann Hw H, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df D, Sullivan M, Kleber K, Ebrahimi R, Xiong S, Brosgart CL. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, von Weizsacker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, Seitz S, Urban S. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol. 2008;26:335–341. doi: 10.1038/nbt1389. [DOI] [PubMed] [Google Scholar]
- Phillips S, Chokshi S, Chatterji U, Riva A, Bobardt M, Williams R, Gallay P, Naoumov NV. Alisporivir inhibition of hepatocyte cyclophilins reduces HBV replication and hepatitis B surface antigen production. Gastroenterology. 2015;148:403–414. e407. doi: 10.1053/j.gastro.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, Schaffner W. A hepatitis B vaccine with a novel adjuvant. Vaccine. 2013;31:5297–5299. doi: 10.1016/j.vaccine.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Reddy KR, Matelich MC, Ugarkar BG, Gomez-Galeno JE, DaRe J, Ollis K, Sun Z, Craigo W, Colby TJ, Fujitaki JM, Boyer SH, van Poelje PD, Erion MD. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J Med Chem. 2008;51:666–676. doi: 10.1021/jm7012216. [DOI] [PubMed] [Google Scholar]
- Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M, Gallagher B, Chao J, Hesselgesser J, Duatschek P, Zheng J, Lu B, Tumas DB, Perry J, Halcomb RL. Identification and optimization of pteridinone Toll-like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J Med Chem. 2013;56:7324–7333. doi: 10.1021/jm400815m. [DOI] [PubMed] [Google Scholar]
- Saruc M, Ozden N, Turkel N, Ayhan S, Hock LM, Tuzcuoglu I, Yuceyar H. Long-term outcomes of thymosin-alpha 1 and interferon alpha-2b combination therapy in patients with hepatitis B e antigen (HBeAg) negative chronic hepatitis B. J Pharm Sci. 2003;92:1386–1395. doi: 10.1002/jps.10401. [DOI] [PubMed] [Google Scholar]
- Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123–132. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Schickli JH, Whitacre DC, Tang RS, Kaur J, Lawlor H, Peters CJ, Jones JE, Peterson DL, McCarthy MP, Van Nest G, Milich DR. Palivizumab epitope-displaying virus-like particles protect rodents from RSV challenge. The Journal of clinical investigation. 2015;125:1637–1647. doi: 10.1172/JCI78450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C, Mason WS. Hepatitis B virus biology. Microbiology and molecular biology reviews : MMBR. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C, Sohn JA. Targeting Hepatitis B Virus With CRISPR/Cas9. Molecular therapy. Nucleic acids. 2014;3:e216. doi: 10.1038/mtna.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigal BA, Connors WH, Fraley A, Borzilleri RM, Carter PH, Emanuel SL, Fargnoli J, Kim K, Lei M, Naglich JG, Pokross ME, Posy SL, Shen H, Surti N, Talbott R, Zhang Y, Terrett NK. The Discovery of Macrocyclic XIAP Antagonists from a DNA-Programmed Chemistry Library, and Their Optimization To Give Lead Compounds with in Vivo Antitumor Activity. J Med Chem. 2015;58:2855–2861. doi: 10.1021/jm501892g. [DOI] [PubMed] [Google Scholar]
- Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, Lam YF, Lie AK, Lai CL, Kwong YL, Yuen MF. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3736–3743. doi: 10.1200/JCO.2014.56.7081. [DOI] [PubMed] [Google Scholar]
- Simsek E, Sinnathamby G, Block TM, Liu Y, Philip R, Mehta AS, Norton PA. Inhibition of cellular alpha-glucosidases results in increased presentation of hepatitis B virus glycoprotein-derived peptides by MHC class I. Virology. 2009;384:12–15. doi: 10.1016/j.virol.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA. Do prodrugs deliver? Curr Opin Drug Discov Devel. 2007;10:550–559. [PubMed] [Google Scholar]
- Springbank . SB 9200 HCV. Spring Bank Parmaceuticals; [Google Scholar]
- Stray SJ, Bourne CR, Punna S, Lewis WG, Finn MG, Zlotnick A. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8138–8143. doi: 10.1073/pnas.0409732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stray SJ, Zlotnick A. BAY 41-4109 has multiple effects on Hepatitis B virus capsid assembly. Journal of molecular recognition : JMR. 2006;19:542–548. doi: 10.1002/jmr.801. [DOI] [PubMed] [Google Scholar]
- Tavis JE, Lomonosova E. The hepatitis B virus ribonuclease H as a drug target. Antiviral research. 2015;118:132–138. doi: 10.1016/j.antiviral.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SL, Sheen IS, Chien RN, Chu CM, Huang HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC, Liaw YF. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10:120–135. doi: 10.1007/BF02256004. [DOI] [PubMed] [Google Scholar]
- Tzeng HT, Tsai HF, Liao HJ, Lin YJ, Chen L, Chen PJ, Hsu PN. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PloS one. 2012;7:e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Uribe LA, O'Brien CG, Wong RJ, Gish RR, Tsai N, Nguyen MH. Current treatment guidelines for chronic hepatitis B and their applications. Journal of clinical gastroenterology. 2014;48:773–783. doi: 10.1097/MCG.0000000000000130. [DOI] [PubMed] [Google Scholar]
- Wang XY, Wei ZM, Wu GY, Wang JH, Zhang YJ, Li J, Zhang HH, Xie XW, Wang X, Wang ZH, Wei L, Wang Y, Chen HS. In vitro inhibition of HBV replication by a novel compound, GLS4, and its efficacy against adefovir-dipivoxil-resistant HBV mutations. Antiviral therapy. 2012;17:793–803. doi: 10.3851/IMP2152. [DOI] [PubMed] [Google Scholar]
- Ward JW. Hepatitis C virus: the 25-year journey from discovery to cure. Hepatology. 2014;60:1479–1482. doi: 10.1002/hep.27377. [DOI] [PubMed] [Google Scholar]
- Weber O, Schlemmer KH, Hartmann E, Hagelschuer I, Paessens A, Graef E, Deres K, Goldmann S, Niewoehner U, Stoltefuss J, Haebich D, Ruebsamen-Waigmann H, Wohlfeil S. Inhibition of human hepatitis B virus (HBV) by a novel non-nucleosidic compound in a transgenic mouse model. Antiviral research. 2002;54:69–78. doi: 10.1016/s0166-3542(01)00216-9. [DOI] [PubMed] [Google Scholar]
- Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney W.E.t., Xiong S, Brosgart CL, Chen SS, Gibbs CS, Zoulim F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. The Journal of clinical investigation. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre DC, Lee BO, Milich DR. Use of hepadnavirus core proteins as vaccine platforms. Expert Rev Vaccines. 2009;8:1565–1573. doi: 10.1586/erv.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, Hegge JO, Klein JJ, Wakefield DH, Oropeza CE, Deckert J, Roehl I, Jahn-Hofmann K, Hadwiger P, Vornlocher HP, McLachlan A, Lewis DL. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Liu B, Zhang Y, Li J, Arzumanyan A, Clayton MM, Schinazi RF, Wang Z, Goldmann S, Ren Q, Zhang F, Feitelson MA. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrobial agents and chemotherapy. 2013;57:5344–5354. doi: 10.1128/AAC.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Jia J, You H. Thymosin alpha-1 treatment in chronic hepatitis B. Expert Opin Biol Ther. 2015:1–4. doi: 10.1517/14712598.2015.1007948. [DOI] [PubMed] [Google Scholar]
- Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DZ, Wang XY, Shen XL, Gong GZ, Ren H, Guo LM, Sun AM, Xu M, Li LJ, Guo XH, Zhen Z, Wang HF, Gong HY, Xu C, Jiang N, Pan C, Gong ZJ, Zhang JM, Shang J, Xu J, Xie Q, Wu TF, Huang WX, Li YG, Xu J, Yuan ZH, Wang B, Zhao K, Wen YM. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. Journal of hepatology. 2013;59:450–456. doi: 10.1016/j.jhep.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, Zhang JM, Xu M, Wang HF, Huang WX, Bai XF, Niu JQ, Liu P, Chen XY, Shen XL, Yuan ZH, Wang XY, Wen YM. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PloS one. 2008;3:e2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, Shen F, Zhang Q, Sun S, Yuan Z. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol Immunol. 2009;46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Lee JH, Kim YJ, Yoon JH, Lee HS. Antiviral efficacy of combination therapy with entecavir and adefovir for entecavir/lamivudine-resistant hepatitis B virus with or without adefovir resistance. Journal of medical virology. 2012;84:424–430. doi: 10.1002/jmv.23229. [DOI] [PubMed] [Google Scholar]
- Yang YF, Zhao W, Zhong YD, Yang YJ, Shen L, Zhang N, Huang P. Comparison of the efficacy of thymosin alpha-1 and interferon alpha in the treatment of chronic hepatitis B: a meta-analysis. Antiviral research. 2008;77:136–141. doi: 10.1016/j.antiviral.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Yapali S, Talaat N, Lok AS. Management of hepatitis B: our practice and how it relates to the guidelines. Clinical gastroenterology and hepatology. 2014;12:16–26. doi: 10.1016/j.cgh.2013.04.036. [DOI] [PubMed] [Google Scholar]
- You J, Zhuang L, Tang BZ, Yang WB, Ding SY, Li W, Wu RX, Zhang HL, Zhang YM, Yan SM, Zhang L. A randomized controlled clinical trial on the treatment of Thymosin a1 versus interferon-alpha in patients with hepatitis B. World journal of gastroenterology : WJG. 2001;7:411–414. doi: 10.3748/wjg.v7.i3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Goddard C, Clearfield E, Mills C, Xiao T, Guo H, Morrey JD, Motter NE, Zhao K, Block TM, Cuconati A, Xu X. Design, synthesis, and biological evaluation of triazolo-pyrimidine derivatives as novel inhibitors of hepatitis B virus surface antigen (HBsAg) secretion. J Med Chem. 2011;54:5660–5670. doi: 10.1021/jm200696v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen MF, Han KH, Um SH, Yoon SK, Kim HR, Kim J, Kim CR, Lai CL. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology. 2010;51:767–776. doi: 10.1002/hep.23462. [DOI] [PubMed] [Google Scholar]
- Yuen MF, Lai CL. Treatment of chronic hepatitis B: Evolution over two decades. Journal of gastroenterology and hepatology. 2011;26(Suppl 1):138–143. doi: 10.1111/j.1440-1746.2010.06545.x. [DOI] [PubMed] [Google Scholar]
- Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, Michel ML, Autran B, Cosset FL, Strick-Marchand H, Trepo C, Kao JH, Carrat F, Lacombe K, Schinazi RF, Barre-Sinoussi F, Delfraissy JF, Zoulim F. Towards an HBV cure: state-ofthe-art and unresolved questions-report of the ANRS workshop on HBV cure. Gut. 2015 doi: 10.1136/gutjnl-2014-308943. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Peng CH, Zheng SS. Programmed death 1 and programmed death ligand 1 expressions in patients with chronic hepatitis B. Hepatobiliary & pancreatic diseases international : HBPD INT. 2013;12:394–399. doi: 10.1016/s1499-3872(13)60061-2. [DOI] [PubMed] [Google Scholar]
- Zoulim F, Durantel D. Antiviral Therapies and Prospects for a Cure of Chronic Hepatitis B. Cold Spring Harbor perspectives in medicine. 2015;5 doi: 10.1101/cshperspect.a021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593–1608. e1591–1592. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- Achillion Pharmaceuticals, I. 2010 http://ir.achillion.com/releasedetail.cfm?releaseid=441884.
- Alnylam Alnylam Announces New RNAi Therapeutic Program for the Treatment of Hepatitis B Virus (HBV) Infection and Reports an Up to 2.3 Log10 Reduction of HBV Surface Antigen (HBsAg) in Chronically Infected Chimpanzees. 2014 http://investors.alnylam.com/releasedetail.cfm?ReleaseID=847055.
- Altravax Hepatitis B Virus (HBV) vaccine. http://www.altravax.com/?page_id=108.
- Arrowhead Arrowhead Cleared to Proceed with Multiple Dose Phase 2b Study of ARC-520. 2015a http://ir.arrowheadresearch.com/releasedetail.cfm?releaseid=906108.
- Benitech Biopharma Hepatitis B. http://www.benitec.com/pipeline/in-house-programs-detail#hepb.
- Contravir CMX157 Overview. http://contravir.com/cmx157/
- DYNAVAX Dynavax Reports Additional Positive Phase 1B Immunogenicity Data for Hepatitis B Therapy Candidate. 2011 http://investors.dynavax.com/releasedetail.cfm?releaseid=551782.
- HEC http://www.hecpharm.com/research-and-development/pipeline.html#.VWiowyj9vKM.
- Hepatera Maxwell Biotech Portfolio Company Hepatera Announces Proof-of-Concept Clinical Results with Myrcludex B, a Novel Entry Inhibitor for Treatment of Chronic Hepatitis B and Delta, PR Newswire. 2014 http://www.prnewswire.com/news-releases/maxwell-biotech-portfolio-company-hepatera-announces-proof-of-concept-clinical-results-with-myrcludex-b-a-novel-entry-inhibitor-for-treatment-of-chronic-hepatitis-b-and-delta-279878112.html.
- Inovio INO-1800 SynCon® immunotherapy for hepatitis B virus. 2015 http://www.inovio.com/products/infectious-disease-vaccines/hepatitis/hbv/
- TetraLogic Birinapant. http://tetralogicpharma.com/birinapant/