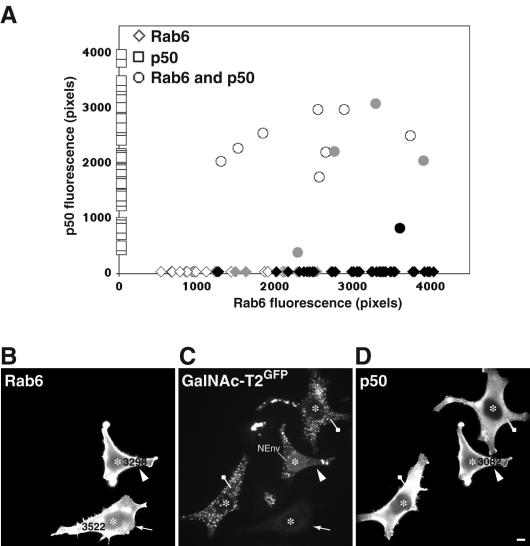
Figure 8.
Disruption of the dynein/dynactin complex inhibits rab6A-GTP-mediated ER accumulation of Golgi enzymes. The experimental procedure is as described in Figure 6, except a p50-encoding expression construct was microinjected into the nucleus of HeLa GalNAc-T2GFP cells. After 24 h of expression, a rab6A-GTP plasmid construct was injected into the same cells. At 7 h postinjection, cells were fixed and processed for indirect immunofluorescence with antibodies against p50 and rab6. (A) Graphical depiction of such an experiment with levels of p50 protein shown by squares on the y-axis, rab6 levels by diamonds on the x-axis, and p50/rab6A-GTP-coexpressing cells by circles. Cells were quantified for the level of rab6 and/or p50 protein and scored for the presence of GaNAc-T2GFP in the juxtanuclear Golgi or in scattered mini-Golgi in the case of p50-expressing cells (open symbols), partially in the ER (gray symbols), or totally localized to the ER (black symbols). Note that p50 overexpression results in the same scattered Golgi phenotype as produced by nocodazole treatment due to inhibition of the dynein motor. (B-D) Morphological validation of the quantitative data in A. HeLa GalNAc-T2GFP cells (C) stained for rab6 (A) and p50 (D). Microinjected cells are labeled with asterisks in each panel. Cells expressing only p50 show the typical Golgi fragmentation phenotype that indicates disruption of the dynein/dynactin complex (drawing pin). At high levels of coexpressed p50 and rab6A-GTP, GalNAc-T2GFP remains in the scattered Golgi (arrowhead), whereas cells expressing equivalent high levels of rab6-GTP only show GaNAc-T2GFP relocalized to the ER (arrow). This figure is representative of three independent experiments. Bar, 10 μm.