Abstract
High milk consumption might shorten life span through increased oxidative stress. We aimed to determine whether higher mortality rates with high milk consumption are modified by fruit and vegetable intake or total antioxidant intake (oxygen radical absorbance capacity). We used information from food frequency questionnaires completed by 61,420 women in a Swedish cohort (22,391 deaths from the 1987–1990 baseline onward), 36,714 women from a second survey (1997) of this cohort, and 45,280 Swedish men (15,478 deaths from the 1998 baseline onward). Compared with low consumption of milk (<1 glass/day) and high consumption of fruits/vegetables (≥5 servings/day), time-updated information revealed an adjusted hazard ratio for death of 2.79 (95% confidence interval (CI): 2.42, 3.21) in women who consumed ≥3 glasses of milk/day and <1 serving/day of fruit/vegetables and a hazard ratio of 1.60 (95% CI: 1.40, 1.82) in women who consumed the same amount of milk but ≥5 servings/day of fruits/vegetables. The same comparisons in men, based on a single food frequency questionnaire, displayed hazard ratios of 1.31 (95% CI: 1.14, 1.51) and 1.07 (95% CI: 0.97, 1.18), respectively. Total antioxidant consumption showed similar patterns as fruit/vegetable intakes. Dietary antioxidant intake, especially in women, seems to modify the elevated death rate associated with high milk consumption.
Keywords: antioxidants, fruit, galactose, lactose, milk, mortality, oxidative stress, vegetables
High milk consumption has long been promoted as strengthening bone and reducing the likelihood of fragility fractures. However, we recently demonstrated a higher risk of fracture with high daily milk consumption in women (1). Mortality rates were also increased in both women and men with high milk consumption. We hypothesized that the underlying mechanism could be explained by the lactose content of milk (1).
Milk is the main dietary source of d-galactose, one component of the disaccharide lactose. Chronic d-galactose exposure in animals, with a dose corresponding to 1–2 glasses of milk in humans (1, 2), is deleterious to health by means of oxidative stress damage and chronic inflammation (2–5). Female animals seem to be especially vulnerable (6–8). The increased oxidative stress with aging and chronic low-grade inflammation is not only a pathogenic mechanism of cardiovascular disease and cancer in humans (9, 10) but also a mechanism of age-related bone loss and sarcopenia (10, 11).
Oxidative stress and inflammation can be reduced by a diet rich in antioxidants (12–15), and such foods could potentially reduce rates of death (16, 17). Because of the antioxidant capacity of vegetables and fruits and the high content of lactose/galactose in milk, which may induce oxidative stress and low-grade inflammation, we hypothesized that a high intake of fruits and vegetables or a high total antioxidant intake (18, 19) may counteract the observed associations of milk intake with mortality. Indeed, experimental evidence in animals indicates that galactose-induced aging can be prevented by a higher intake of fruits and vegetables (20–24).
To our knowledge, no previous clinical study has combined milk consumption with fruit and vegetable intake and total antioxidant intake to evaluate associations with the rate of death. In Scandinavia, consumption of milk and of fruits and vegetables displays a wide range in intake (1, 25, 26). Therefore, our main objective in this Swedish cohort study was to determine whether fruit and vegetable intake or total antioxidant intake modifies the previously observed relationship between milk consumption and death.
METHODS
We used data from 2 previously described (1) population-based cohort studies, the Swedish Mammography Cohort (SMC) and the Cohort of Swedish Men. The SMC started in 1987–1990 when 74% of all 90,303 women aged 39–74 years residing in 2 Swedish counties completed a questionnaire covering diet (food frequency questionnaire (FFQ)) and lifestyle that had been enclosed with a mailed invitation to undergo routine mammography screening. In 1997, a subsequent expanded questionnaire was sent to the 56,030 women still living in the study area (response rate 70%). We excluded women with implausible values for total energy intake (≥3 standard deviations below or above the log-transformed mean energy intake; cutoffs were 574 kcal/day and 4,707 kcal/day) (27) and those with missing data on all items regarding fruit and vegetable consumption. Exclusion of outliers for energy intake, in addition to adjustment for total energy intake in the statistical analyses, compensates for overall under- or overreporting of dietary intake (28). In the present study, a first analysis included 61,240 women without a prevalent cancer diagnosis in the SMC with information from 1987–1990 and 38,331 women with updated information from 1997. In a second analysis with baseline set at the second examination, we included 36,714 women who were alive on January 1, 1998, and free of any previous cancers.
The Cohort of Swedish Men was established in 1997. All men aged 45–79 years residing in 2 counties in central Sweden were invited to participate in the study (n = 100,303). The FFQ and lifestyle questionnaire was completed by 48,850 men. Despite a response rate of only 49%, the Cohort of Swedish Men is representative of Swedish men in this age range in relation to age distribution, educational level, and prevalence of overweight (29). We excluded men with implausible values for total energy intake (cutoffs were 861 kcal/day and 7,311 kcal/day). For the present analysis, 45,280 men who were alive on January 1, 1998, and free of previous cancers were available.
The single FFQ administered in 1997 was used to simplify the comparison between men and women, but in women we also used time-updated information by means of the complete SMC data set. The studies have been approved by the Regional Ethical Review Board in Stockholm, Sweden.
Exposures
The participants reported, by means of a valid and reproducible FFQ, their average frequency of consumption of up to 96 foods and beverages during the past year, including milk (either low-fat (≤0.5%), medium-fat (1.5%), or high-fat (3%)), sour milk, yogurt, cheese, 5 fruits (apples, bananas, berries, oranges/citrus fruit, and other fruit), orange juice, and 13 vegetables (carrots, beet root, broccoli, cabbage, cauliflower, lettuce, onion, garlic, peas, pea soup, peppers, spinach, tomatoes, and other vegetables) (29–31). There were 8 possible frequency categories in increasing order from zero times per month to more than 3 times per day. In the 1987–1990 FFQ, the numbers of fruit (n = 4) and vegetable (n = 5) categories were fewer, but they comprised more fruit or vegetable items in each category. The fruit and vegetable categories represented the typical consumption pattern in Sweden at the time of each investigation, with a higher number of items over time. In accordance with national dietary guidelines (32), only 1 glass of juice (fresh or from concentrate) was included in the calculation of daily intake, independent of the amount ingested. Instructions in the FFQ stated that 1 serving of milk corresponds to 1 glass of 200 mL. Milk intake was specified according to fat content, and intakes were summed into a single measure representing total milk intake on a continuous scale. Missing values for individual dairy products were interpreted as no intake of that particular food (33). The small fraction of missing data for single items, which were regarded as zero consumption, is unlikely to represent a bias for the observed findings (33). In fact, 92% of those who did not report milk consumption on the FFQ part of the questionnaire reported that they did not consume milk when posed a specific question, and 99.8% had consumption of less than 1 glass/day according to complementary open questions regarding dairy consumption.
Nutrient intakes were estimated by multiplying the consumption frequency of each food item by the nutrient content of age-specific portion sizes and reference data obtained from the Swedish National Food Agency database (34) and were adjusted for total energy intake using the residual method (28). According to validation studies of the self-reported milk intakes, the Spearman coefficient for correlation between the FFQ and four 7-day food records every third month (a gold standard reference) was approximately 0.7 (35). Spearman coefficients for correlation between the FFQ and the averages of these four 7-day dietary records ranged between 0.4 and 0.7 for individual fruit and vegetable items.
We calculated estimates of total antioxidant capacity from diet analyzed with an oxygen radical absorbance capacity (ORAC) assay, as described in detail previously (18). The FFQ contained 31 items with available ORAC values. The total antioxidant capacity of the diet (µmol/day) was calculated as the sum of the antioxidant content of the 31 food items, calculated by multiplying the average daily consumption of each food by its ORAC concentration (μmol Trolox equivalents (TE)/100 g) (Trolox: F. Hoffmann-La Roche AG, Basel, Switzerland). Because antioxidants in coffee and tea have been shown to be poorly absorbed, we took into account absorption (6% for coffee and 4% for tea) when calculating the ORAC (36). The correlation between the total antioxidant capacities of dietary ORAC and plasma ORAC was 0.31.
Outcomes
We considered as the primary outcome all-cause mortality registered between baseline and September 30, 2015, in the Swedish Cause of Death Registry. We used the underlying cause of death from the Swedish Cause of Death Registry to define secondary outcomes: mortality from cardiovascular diseases (International Classification of Diseases, Tenth Revision, codes I00–I99) and cancer (International Classification of Diseases, Tenth Revision, C-codes) through December 31, 2014. For 1987–1996, we used corresponding codes from the International Classification of Diseases, Ninth Revision.
Statistical analysis
We calculated time at risk for each participant from study entry until the date of each outcome, the date of emigration, or the end of the study period, whichever came first. We first evaluated trends in mortality rates according to milk intake, fruit and vegetable intake, and ORAC using restricted cubic-spline Cox regression with 3 knots placed at percentiles 10, 50, and 90 of the exposures (37). We calculated age-adjusted death rates and age- and multivariable-adjusted hazard ratios and 95% confidence intervals for categories of milk intake (<1, 1–<2, 2–<3, or ≥3 glasses/day) and categories of fruit and vegetable intake or quartiles of ORAC. We categorized fruit and vegetable intake as <1, 1–<2, 2–<3, 3–<4, 4–<5, or ≥5 servings/day, with the latter category reflecting dietary recommendations (32). The proportional hazards assumptions were confirmed graphically by log-log plots.
To select suitable covariates for the multivariable model, we used present knowledge and directed acyclic graphs (38). The model for the total effect included age, total energy intake, body mass index (weight (kg)/height (m)2), height, intakes of yogurt, cheese, red and processed meat, and alcohol (all continuous), educational level (≤9 years, 10–12 years, >12 years, or other), living alone (yes/no), ever use of antioxidant supplements (yes/no), physical activity (metabolic equivalent-hours/day; continuous), smoking status (never, former with <20 pack-years, former with ≥20 pack-years, current with <20 pack-years, or current with ≥20 pack-years) and Charlson's comorbidity index (possible range of scores, 0–33; continuous) (39, 40). To avoid loss of efficiency and limit the introduction of bias by restricting the analysis to persons with complete data alone, missing data on covariates were imputed using multiple imputation (41). We also imputed covariates not assessed at the baseline of the SMC in 1987–1990 (e.g., smoking status and physical activity) (1). Additional sensitivity analyses included exclusion of the first 2 years of follow-up, persons with a body mass index greater than 35, and current smokers. To our second model, we also added as covariates use of calcium-containing supplements and, for baseline 1997, use of aspirin and prevalent hypercholesterolemia. In a fourth model, we additionally adjusted our estimates for energy-adjusted dietary intakes of protein, total and saturated fat, calcium, vitamin D, retinol, and phosphorus; ever use of cortisone; and, among women, hormone replacement therapy.
Measures of interaction were calculated on the basis of adjusted hazard ratios (HRs), using persons consuming less than 1 glass of milk and 5 or more servings of fruit and vegetables per day as the reference category for the following groups (annotations in parentheses): milk intake ≥3 glasses/day, fruit and vegetable intake ≥5 servings/day (HR10); milk intake <1 glass/day, fruit and vegetable intake <1 serving/day (HR01); and milk intake ≥3 glasses/day, fruit and vegetable intake <1 serving/day (HR11). The relative excess risk of interaction (interaction on the additive scale) was calculated as HR11 − HR10 − HR01 + 1 (42), and 95% confidence intervals were obtained by means of the bootstrap percentile method with 1,000 bootstrap samples. The statistical analyses were performed with Stata 13.1 (StataCorp LP, College Station, Texas).
RESULTS
Characteristics of the study population by baseline date and sex are presented in Tables 1–3. Approximately 9% of the women reported milk consumption of ≥3 glasses/day in 1987–1990 (Table 1), whereas in 1997 only 2% reported such intake (Table 2). Men had on average higher consumption of milk; 15% drank ≥3 glasses/day in 1997 (Table 3). Average reported consumption of fruits and vegetables among women was 3.5 servings/day (standard deviation (SD), 2.0) at baseline and 5.3 servings/day (SD, 3.0) at the follow-up examination. Men reported an average consumption of 4.1 servings/day (SD, 2.5). Intake of fruits and vegetables did not vary by milk intake in either women or men.
Table 1.
Characteristics of the Swedish Mammography Cohort (Women) According to Milk Consumption at the 1987–1990 Baseline (n = 61,240)
| Characteristic | Milk Intake, glasses/day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–<2 | 2–<3 | ≥3 | |||||||||
| Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | |
| All participants | 16,869 | 27.5 | 23,385 | 38.2 | 15,405 | 25.2 | 5,581 | 9.1 | ||||
| Age at entry, years | 53.2 (9.5) | 54.0 (9.7) | 54.1 (9.9) | 52.8 (9.6) | ||||||||
| Body mass indexa (n = 58,960) | 24.4 (3.9) | 24.7 (3.8) | 25.0 (4.0) | 24.9 (4.2) | ||||||||
| Height, m (n = 59,685) | 1.64 (0.06) | 1.64 (0.06) | 1.64 (0.06) | 1.64 (0.06) | ||||||||
| Dietary intake | ||||||||||||
| Energy, kcal/day | 1,414 (433) | 1,537 (413) | 1,709 (433) | 1,967 (525) | ||||||||
| Milk, g/dayb | 17.3 (37.3) | 201.6 (14.9) | 400.2 (6.0) | 676.8 (151.9) | ||||||||
| Yogurt, g/day | 102.7 (117.3) | 97.8 (101.2) | 93.4 (105.1) | 87.2 (113.1) | ||||||||
| Cheese, g/day | 26.8 (21.2) | 26.3 (19.6) | 26.8 (19.8) | 27.7 (22.0) | ||||||||
| Fruit, g/day (n = 61,031) | 201.0 (143.4) | 196.6 (132.5) | 191.6 (136.7) | 183.0 (147.1) | ||||||||
| Vegetables, g/day (n = 61,104) | 92.5 (67.5) | 87.6 (60.2) | 85.7 (63.8) | 81.7 (63.9) | ||||||||
| Fruit and vegetables, servings/day | 3.6 (2.1) | 3.5 (1.9) | 3.4 (2.0) | 3.2 (2.1) | ||||||||
| Red and processed meat, g/day | 70.2 (42.2) | 75.5 (39.8) | 79.8 (40.6) | 85.4 (44.8) | ||||||||
| Protein, g/day | 62.3 (9.1) | 66.4 (8.0) | 69.9 (8.2) | 73.1 (8.8) | ||||||||
| Total fat, g/day | 44.3 (17.0) | 48.9 (16.7) | 55.0 (18.4) | 65.0 (24.2) | ||||||||
| Saturated fat, g/day | 19.0 (8.1) | 21.4 (8.0) | 24.5 (9.1) | 30.0 (12.6) | ||||||||
| Calcium, mg/day | 733 (159) | 859 (139) | 972 (144) | 1,101 (174) | ||||||||
| Vitamin D, μg/day | 3.9 (1.3) | 4.4 (1.2) | 4.8 (1.4) | 5.1 (1.7) | ||||||||
| Phosphorus, mg/day | 1,242 (184) | 1,365 (165) | 1,478 (174) | 1,587 (204) | ||||||||
| Retinol, mg/day | 0.94 (0.70) | 1.03 (0.64) | 1.09 (0.61) | 1.09 (0.59) | ||||||||
| Alcohol, g/day | 3.1 (4.0) | 2.6 (3.5) | 2.1 (3.0) | 1.9 (3.0) | ||||||||
| Physical activity, MET-hours/dayc | 42.2 (4.8) | 42.4 (4.8) | 42.6 (4.8) | 42.8 (5.0) | ||||||||
| Leisure-time physical activity, hours/weekc | ||||||||||||
| <1 | 3,256 | 19.3 | 4,396 | 18.8 | 2,900 | 18.8 | 1,077 | 19.3 | ||||
| 1 | 4,254 | 25.2 | 6,094 | 26.1 | 3,954 | 25.7 | 1,429 | 25.6 | ||||
| 2–3 | 5,282 | 31.3 | 7,526 | 32.2 | 4,900 | 31.8 | 1,783 | 31.9 | ||||
| 4–5 | 2,423 | 14.4 | 3,286 | 14.1 | 2,301 | 14.9 | 783 | 14.0 | ||||
| >5 | 1,654 | 9.8 | 2,083 | 8.9 | 1,350 | 8.8 | 509 | 9.1 | ||||
| Education, years | ||||||||||||
| ≤9 | 13,235 | 78.5 | 18,676 | 79.9 | 12,487 | 81.1 | 4,393 | 78.7 | ||||
| 10–12 | 1,366 | 8.1 | 1,699 | 7.3 | 1,054 | 6.8 | 403 | 7.2 | ||||
| >12 | 897 | 5.3 | 1,146 | 4.9 | 632 | 4.1 | 266 | 4.8 | ||||
| Otherd | 1,371 | 8.1 | 1,864 | 8.0 | 1,232 | 8.0 | 519 | 9.3 | ||||
| Smoking statusc | ||||||||||||
| Never smoker | 8,206 | 48.6 | 12,221 | 52.3 | 7,870 | 51.1 | 2,595 | 46.5 | ||||
| Former smoker | 5,318 | 31.5 | 6,875 | 29.4 | 4,450 | 28.9 | 1,646 | 29.5 | ||||
| Current smoker | 3,345 | 19.8 | 4,289 | 18.3 | 3,085 | 20.0 | 1,340 | 24.0 | ||||
| Living alone | 3,939 | 23.4 | 5,292 | 22.6 | 3,741 | 24.3 | 1,430 | 25.6 | ||||
| Charlson comorbidity indexe | ||||||||||||
| 0 | 15,143 | 89.8 | 21,111 | 90.3 | 13,817 | 89.7 | 4,907 | 87.9 | ||||
| 1 | 1,401 | 8.3 | 1,791 | 7.7 | 1,231 | 8.0 | 528 | 9.5 | ||||
| ≥2 | 325 | 1.9 | 483 | 2.1 | 357 | 2.3 | 146 | 2.6 | ||||
| Current use of calcium-containing supplements (regular or occasional)c | 2,914 | 17.3 | 3,840 | 16.4 | 2,159 | 14.0 | 801 | 14.4 | ||||
| Ever use of antioxidant-containing supplementsc | 5,027 | 29.8 | 6,700 | 28.7 | 3,895 | 25.3 | 1,348 | 24.2 | ||||
| Ever use of estrogen replacement therapyc | 3,495 | 20.7 | 5,063 | 21.7 | 3,508 | 22.8 | 1,458 | 26.1 | ||||
| Ever use of cortisonec | 897 | 5.3 | 1,057 | 4.5 | 733 | 4.8 | 256 | 4.6 | ||||
Abbreviations: MET, metabolic equivalent; SD, standard deviation.
a Weight (kg)/height (m)2.
b 1 g/day of milk ≈ 1 mL/day.
c Values were imputed from the 1997 questionnaire.
d Such as vocational education.
eInternational Classification of Diseases (Eighth, Ninth, and Tenth revisions) diagnosis codes were collated from the National Patient Registry to calculate weighted Charlson comorbidity scores. The Charlson comorbidity index predicts mortality for a patient who may have a range of up to 17 comorbid conditions. Each condition is assigned a score of 1–6, depending on the risk of dying associated with the condition.
Table 3.
Characteristics of the Cohort of Swedish Men (Men) According to Milk Consumption at the 1997 Baseline (n = 45,280)
| Characteristic | Milk Intake, glasses/day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–<2 | 2–<3 | ≥3 | |||||||||
| Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | |
| All participants | 20,144 | 44.5 | 10,803 | 23.9 | 7,459 | 16.5 | 6,874 | 15.2 | ||||
| Age at entry, years | 59.5 (9.6) | 61.2 (9.9) | 61.1 (9.8) | 60.1 (9.5) | ||||||||
| Body mass indexa (n = 42,975) | 25.6 (3.2) | 25.6 (3.3) | 25.9 (3.4) | 26.4 (3.6) | ||||||||
| Height, m (n = 43,190) | 1.77 (0.07) | 1.77 (0.07) | 1.77 (0.07) | 1.77 (0.07) | ||||||||
| Dietary intake | ||||||||||||
| Energy, kcal/day | 2,511 (789) | 2,583 (772) | 2,749 (798) | 3,129 (914) | ||||||||
| Milk, g/dayb | 64.7 (68.3) | 267.5 (59.4) | 467.3 (49.3) | 909.1 (372.4) | ||||||||
| Yogurt, g/day | 181.4 (237.6) | 165.7 (222.1) | 177.9 (245.2) | 185.6 (286.8) | ||||||||
| Cheese, g/day | 72.8 (59.8) | 70.6 (54.9) | 75.0 (57.7) | 85.8 (66.1) | ||||||||
| Fruit, g/day (n = 44,870) | 182.1 (140.6) | 182.5 (133.0) | 173.8 (137.3) | 162.1 (131.3) | ||||||||
| Vegetables, g/day (n = 45,110) | 184.2 (125.0) | 181.2 (123.6) | 173.0 (118.3) | 163.5 (118.7) | ||||||||
| Fruit and vegetables, servings/day | 4.2 (2.5) | 4.2 (2.4) | 3.9 (2.4) | 3.7 (2.3) | ||||||||
| Oxygen radical absorbance capacity, µmol/day | 14,218 (5,376) | 14,740 (5,354) | 14,834 (5,563) | 15,153 (5,708) | ||||||||
| Red and processed meat, g/day | 101.9 (63.8) | 102.3 (63.8) | 103.3 (59.2) | 108.8 (61.4) | ||||||||
| Protein, g/day | 98.3 (14.7) | 102.1 (13.9) | 105.4 (14.0) | 111.3 (15.1) | ||||||||
| Total fat, g/day | 89.6 (15.5) | 89.3 (14.6) | 89.2 (14.9) | 89.0 (15.6) | ||||||||
| Saturated fat, g/day | 40.3 (9.5) | 40.6 (9.1) | 41.2 (9.5) | 42.0 (10.0) | ||||||||
| Calcium, mg/day | 1,247 (386) | 1,450 (370) | 1,640 (386) | 1,945 (483) | ||||||||
| Vitamin D, μg/day | 6.1 (3.0) | 6.7 (2.9) | 7.0 (2.9) | 7.7 (3.0) | ||||||||
| Phosphorus, mg/day | 1,922 (294) | 2,063 (282) | 2,185 (292) | 2,387 (350) | ||||||||
| Retinol, mg/day | 1.19 (0.96) | 1.25 (0.85) | 1.28 (0.71) | 1.35 (0.74) | ||||||||
| Alcohol, g/day (n = 40,493) | 17.0 (22.9) | 13.7 (17.6) | 13.1 (18.7) | 14.2 (26.8) | ||||||||
| Physical activity, MET-hours/day (n = 34,812) | 41.2 (4.8) | 41.5 (4.8) | 41.8 (5.0) | 42.3 (5.3) | ||||||||
| Leisure-time physical activity, hours/week (n = 40,496) | ||||||||||||
| <1 | 3,988 | 22.1 | 1,940 | 20.0 | 1,452 | 21.7 | 1,488 | 24.6 | ||||
| 1 | 3,470 | 19.2 | 1,845 | 19.0 | 1,295 | 19.4 | 1,078 | 17.9 | ||||
| 2–3 | 5,691 | 31.5 | 3,127 | 32.2 | 2,064 | 30.9 | 1,792 | 29.7 | ||||
| 4–5 | 2,315 | 12.8 | 1,256 | 12.9 | 876 | 13.1 | 728 | 12.1 | ||||
| >5 | 2,592 | 14.4 | 1,547 | 15.9 | 999 | 14.9 | 953 | 15.8 | ||||
| Education, years (n = 45,129) | ||||||||||||
| ≤9 | 13,072 | 65.1 | 7,437 | 69.0 | 5,502 | 74.1 | 5,308 | 77.5 | ||||
| 10–12 | 3,151 | 15.7 | 1,526 | 14.2 | 884 | 11.9 | 710 | 10.4 | ||||
| >12 | 3,775 | 18.8 | 1,764 | 16.4 | 1,021 | 13.7 | 800 | 11.7 | ||||
| Otherc | 80 | 0.4 | 46 | 0.4 | 23 | 0.3 | 30 | 0.4 | ||||
| Smoking status (n = 44,858) | ||||||||||||
| Never smoker | 6,996 | 35.0 | 4,152 | 38.7 | 2,718 | 36.9 | 2,301 | 33.8 | ||||
| Former smoker | 8,191 | 41.0 | 4,028 | 37.6 | 2,694 | 36.6 | 2,488 | 36.6 | ||||
| <20 pack-yearsd | 4,884 | 25.8 | 2,381 | 23.6 | 1,535 | 22.3 | 1,337 | 21.0 | ||||
| ≥20 pack-years | 2,656 | 14.1 | 1,277 | 12.6 | 895 | 13.0 | 900 | 14.1 | ||||
| Current smoker | 4,786 | 24.0 | 2,539 | 23.7 | 1,954 | 26.5 | 2,011 | 29.6 | ||||
| <20 pack-years | 1,763 | 9.3 | 933 | 9.2 | 712 | 10.3 | 653 | 10.2 | ||||
| ≥20 pack-years | 2,599 | 13.8 | 1,352 | 13.4 | 1,027 | 14.9 | 1,188 | 18.6 | ||||
| Living alone | 3,317 | 16.5 | 1,831 | 16.9 | 1,311 | 17.6 | 1,376 | 20.0 | ||||
| Charlson comorbidity indexe | ||||||||||||
| 0 | 17,102 | 84.9 | 8,892 | 82.3 | 6,182 | 82.9 | 5,667 | 82.4 | ||||
| 1 | 2,152 | 10.7 | 1,314 | 12.2 | 883 | 11.8 | 838 | 12.2 | ||||
| ≥2 | 890 | 4.4 | 597 | 5.5 | 394 | 5.3 | 369 | 5.4 | ||||
| Current use of calcium-containing supplements (regular or occasional) | 2,912 | 14.5 | 1,526 | 14.1 | 976 | 13.1 | 837 | 12.2 | ||||
| Ever use of antioxidant-containing supplements | 6,395 | 31.7 | 3,380 | 31.3 | 2,250 | 30.2 | 1,978 | 28.8 | ||||
| Ever use of aspirin | 6,500 | 32.3 | 3,585 | 33.2 | 2,397 | 32.1 | 2,255 | 32.8 | ||||
| Ever hypercholesterolemiaf | 8,967 | 44.5 | 4,704 | 43.5 | 3,221 | 43.2 | 3,145 | 45.8 | ||||
| Ever use of cortisone | 843 | 4.2 | 449 | 4.2 | 309 | 4.1 | 310 | 4.5 | ||||
Abbreviations: MET, metabolic equivalent; SD, standard deviation.
a Weight (kg)/height (m)2.
b 1 g/day of milk ≈ 1 mL/day.
c Such as vocational education.
d Information on pack-years of smoking was available for 42,259 participants.
eInternational Classification of Diseases (Eighth, Ninth, and Tenth revisions) diagnosis codes were collated from the National Patient Registry to calculate weighted Charlson comorbidity scores. The Charlson comorbidity index predicts mortality for a patient who may have a range of up to 17 comorbid conditions. Each condition is assigned a score of 1–6, depending on the risk of dying associated with the condition.
f High cholesterol or ever use of statins.
Table 2.
Characteristics of the Swedish Mammography Cohort (Women) According to Milk Consumption at the 1997 Baseline (n = 36,714)
| Characteristic | Milk Intake, glasses/day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–<2 | 2<3 | 3 | |||||||||
| Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | |
| All participants | 26,617 | 72.5 | 7,282 | 19.8 | 2,157 | 5.9 | 658 | 1.8 | ||||
| Age at entry, years | 61.2 (9.2) | 63.4 (9.3) | 63.2 (9.1) | 63.0 (9.5) | ||||||||
| Body mass indexa (n = 36,076) | 24.9 (3.9) | 25.4 (4.0) | 25.6 (4.2) | 25.4 (4.1) | ||||||||
| Height, m (n = 36,551) | 1.65 (0.06) | 1.64 (0.06) | 1.65 (0.06) | 1.65 (0.06) | ||||||||
| Dietary intake | ||||||||||||
| Energy, kcal/day | 1,676 (504) | 1,827 (521) | 2,005 (561) | 2,271 (651) | ||||||||
| Milk, g/dayb | 75.6 (60.6) | 277.2 (34.4) | 465.0 (65.1) | 908.7 (307.8) | ||||||||
| Yogurt, g/day | 173.7 (194.8) | 182.1 (209.4) | 195.3 (250.0) | 276.3 (409.6) | ||||||||
| Cheese, g/day | 49.3 (40.0) | 50.4 (37.6) | 55.6 (42.5) | 59.6 (51.8) | ||||||||
| Fruit, g/day (n = 36,450) | 227.8 (155.3) | 218.7 (152.8) | 209.9 (149.9) | 223.9 (176.2) | ||||||||
| Vegetables, g/day (n = 36,628) | 219.4 (144.8) | 211.6 (141.0) | 208.4 (135.2) | 213.5 (148.6) | ||||||||
| Fruit and vegetables, servings/day | 5.4 (3.0) | 5.1 (2.9) | 5.0 (2.8) | 5.2 (3.1) | ||||||||
| Oxygen radical absorbance capacity, µmol/day | 12,841 (5,030) | 13,225 (5,254) | 13,374 (5,106) | 13,803 (5,865) | ||||||||
| Red and processed meat, g/day | 63.8 (45.0) | 67.7 (46.1) | 70.8 (50.4) | 68.3 (51.0) | ||||||||
| Protein, g/day | 69.5 (11.4) | 72.8 (11.0) | 75.3 (11.2) | 81.2 (11.9) | ||||||||
| Total fat, g/day | 60.1 (10.4) | 59.1 (9.8) | 58.5 (10.2) | 56.2 (10.8) | ||||||||
| Saturated fat, g/day | 27.3 (6.4) | 27.1 (6.1) | 27.1 (6.3) | 26.9 (6.6) | ||||||||
| Calcium, mg/day | 977 (270) | 1,157 (268) | 1,297 (288) | 1,618 (383) | ||||||||
| Vitamin D, μg/day | 4.3 (1.6) | 4.9 (1.5) | 5.4 (1.5) | 6.0 (1.8) | ||||||||
| Phosphorus, μg/day | 1,356 (211) | 1,488 (210) | 1,581 (219) | 1,800 (280) | ||||||||
| Retinol, mg/day | 0.86 (0.68) | 0.95 (0.73) | 0.98 (0.73) | 1.00 (0.49) | ||||||||
| Alcohol, g/day | 4.5 (5.4) | 3.2 (4.5) | 2.9 (4.3) | 3.8 (7.2) | ||||||||
| Physical activity, MET-hours/day (n = 28,321) | 42.3 (4.7) | 42.8 (4.8) | 42.6 (5.0) | 42.7 (5.4) | ||||||||
| Leisure-time physical activity, hours/week (n = 32,799) | ||||||||||||
| <1 | 4,687 | 19.6 | 1,285 | 19.8 | 375 | 19.8 | 118 | 21.0 | ||||
| 1 | 5,729 | 24.0 | 1,469 | 22.7 | 428 | 22.6 | 116 | 20.7 | ||||
| 2–3 | 8,045 | 33.7 | 2,220 | 34.3 | 635 | 33.5 | 170 | 30.3 | ||||
| 4–5 | 2,773 | 11.6 | 797 | 12.3 | 228 | 12.0 | 71 | 12.7 | ||||
| >5 | 2,633 | 11.0 | 703 | 10.9 | 231 | 12.2 | 86 | 15.3 | ||||
| Education, years (n = 36,683) | ||||||||||||
| ≤9 | 19,126 | 71.9 | 5,759 | 79.2 | 1,721 | 79.8 | 483 | 73.4 | ||||
| 10–12 | 2,133 | 8.0 | 465 | 6.4 | 115 | 5.3 | 50 | 7.6 | ||||
| >12 | 5,294 | 19.9 | 1,035 | 14.2 | 311 | 14.4 | 123 | 18.7 | ||||
| Otherc | 40 | 0.2 | 16 | 0.2 | 10 | 0.5 | 2 | 0.3 | ||||
| Smoking status (n = 36,182) | ||||||||||||
| Never smoker | 13,804 | 52.7 | 4,134 | 57.6 | 1,157 | 54.2 | 333 | 51.2 | ||||
| Former smoker | 6,348 | 24.2 | 1,384 | 19.3 | 395 | 18.5 | 150 | 23.0 | ||||
| <20 pack-yearsd | 4,910 | 19.2 | 1,038 | 14.8 | 277 | 13.3 | 115 | 18.1 | ||||
| ≥20 pack-years | 1,069 | 4.2 | 255 | 3.6 | 81 | 3.9 | 26 | 4.1 | ||||
| Current smoker | 6,064 | 23.1 | 1,662 | 23.1 | 583 | 27.3 | 168 | 25.8 | ||||
| <20 pack-years | 3,328 | 13.0 | 886 | 12.6 | 297 | 14.3 | 74 | 11.7 | ||||
| ≥20 pack-years | 2,460 | 9.6 | 700 | 10.0 | 268 | 12.9 | 86 | 13.6 | ||||
| Living alone | 5,580 | 21.0 | 1,691 | 23.2 | 504 | 23.4 | 172 | 26.1 | ||||
| Charlson comorbidity indexe | ||||||||||||
| 0 | 23,416 | 88.0 | 6,290 | 86.4 | 1,857 | 86.1 | 541 | 82.2 | ||||
| 1 | 2,163 | 8.1 | 668 | 9.2 | 219 | 10.2 | 74 | 11.2 | ||||
| ≥2 | 1,038 | 3.9 | 324 | 4.4 | 81 | 3.8 | 43 | 6.5 | ||||
| Current use of calcium-containing supplements (regular or occasional) | 6,465 | 24.3 | 1,735 | 23.8 | 529 | 24.5 | 160 | 24.3 | ||||
| Ever use of antioxidant-containing supplements | 11,764 | 44.2 | 3,174 | 43.6 | 930 | 43.1 | 288 | 43.8 | ||||
| Ever use of aspirin | 11,495 | 43.2 | 3,132 | 43.0 | 960 | 44.5 | 285 | 43.3 | ||||
| Ever hypercholesterolemiaf | 9,520 | 35.8 | 2,699 | 37.1 | 771 | 35.7 | 216 | 32.8 | ||||
| Ever use of estrogen replacement therapy | 12,440 | 47.5 | 3,115 | 43.5 | 906 | 42.7 | 313 | 48.5 | ||||
| Ever use of cortisone | 1,929 | 7.2 | 550 | 7.6 | 196 | 9.1 | 52 | 7.9 | ||||
Abbreviations: MET, metabolic equivalent; SD, standard deviation.
a Weight (kg)/height (m)2.
b 1 g/day of milk ≈ 1 mL/day.
c Such as vocational education.
d Information on pack-years of smoking was available for 35,298 participants.
eInternational Classification of Diseases (Eighth, Ninth, and Tenth revisions) diagnosis codes were collated from the National Patient Registry to calculate weighted Charlson comorbidity scores. The Charlson comorbidity index predicts mortality for a patient who may have a range of up to 17 comorbid conditions. Each condition is assigned a score of 1–6, depending on the risk of dying associated with the condition.
f High cholesterol or ever use of statins.
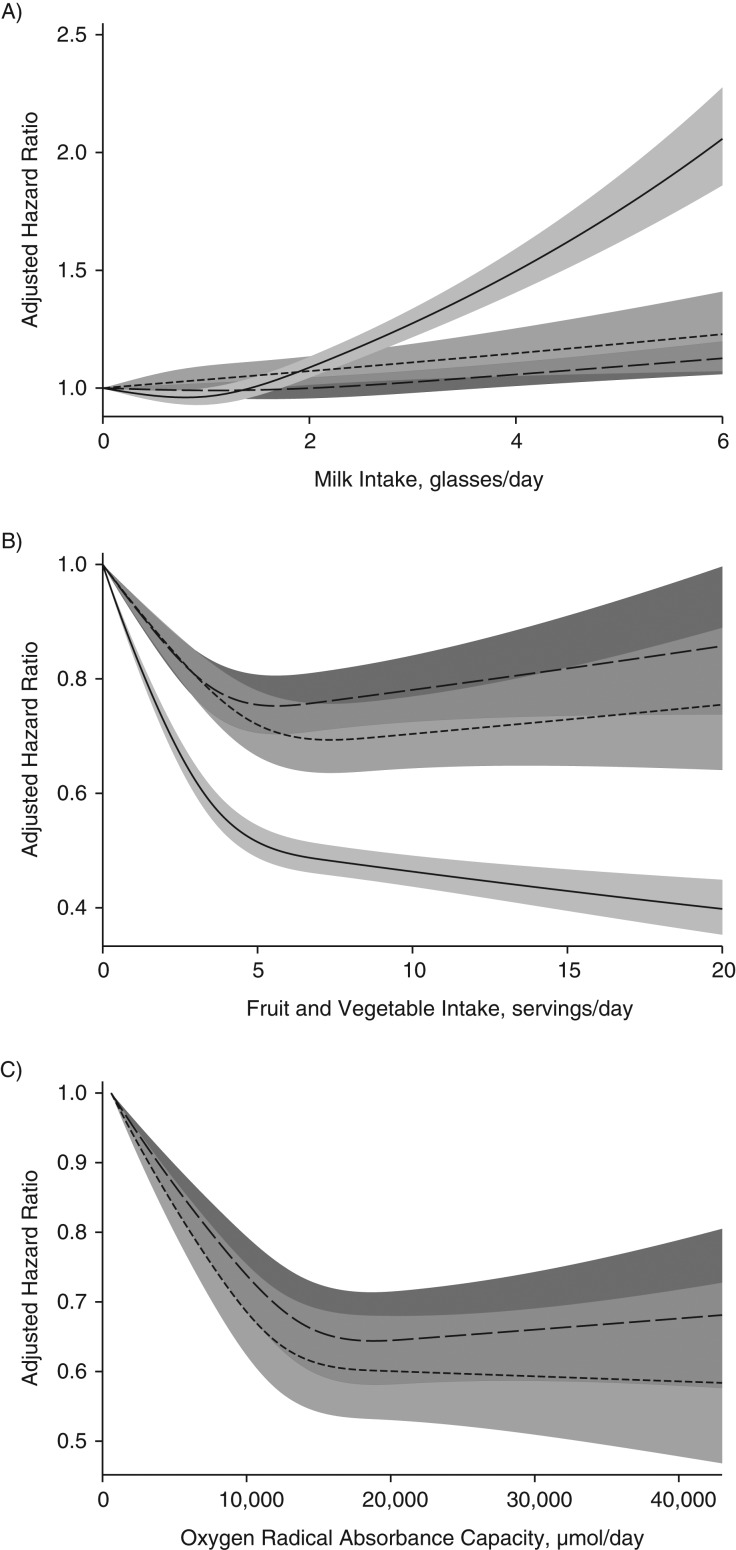
During a mean follow-up period of 23 years (maximum 29 years), 22,391 women (total time at risk = 1,434,171 person-years) died. From January 1, 1998, onward, 10,314 women (581,785 person-years) and 15,478 men (687,688 person-years) died during a mean follow-up period of 15 years (maximum 17 years). Death rates increased in both sexes with increasing milk consumption (Figure 1A), and death rates decreased with higher consumption of fruit and vegetables (Figure 1B), as well as with higher ORACs (Figure 1C), although a threshold seemed to be discerned (>5 servings of fruit/vegetables per day and 15,000 µmol ORAC/day). In men, higher death rates started to be observed after only 3 or more glasses of milk per day (Figure 1A). In women, death rates were already increased at 1–2 glasses of milk per day.
Figure 1.
Sex-specific multivariable-adjusted spline curves illustrating the relationship of milk intake (A), fruit and vegetable intake (B), and oxygen radical absorbance capacity (ORAC; µmol/day) (C) with hazard ratios for death from all causes by the use of time-updated information in the whole Swedish Mammography Cohort (SMC; baseline 1987–1990) (solid line), in the SMC after administration of the second food frequency questionnaire (baseline 1997) (short-dashed line), and in the Cohort of Swedish Men (baseline 1998) (long-dashed line). The shaded areas illustrate 95% confidence intervals. One glass of milk corresponds to 200 mL. Covariates were age, body mass index (weight (kg)/height (m)2), height, energy intake, alcohol intake, intakes of yogurt, cheese, and red and processed meat, education, marital status (living alone vs. not), physical activity (metabolic equivalent-hours/day), smoking habits (never, former, or current smoker and, for baseline 1997, also pack-years of smoking), ever use of antioxidant-containing supplements, and weighted Charlson's comorbidity index. Associations with milk intake were further adjusted for intake of fruit and vegetables, and associations with fruit and vegetable intake and ORAC were adjusted for intake of milk.
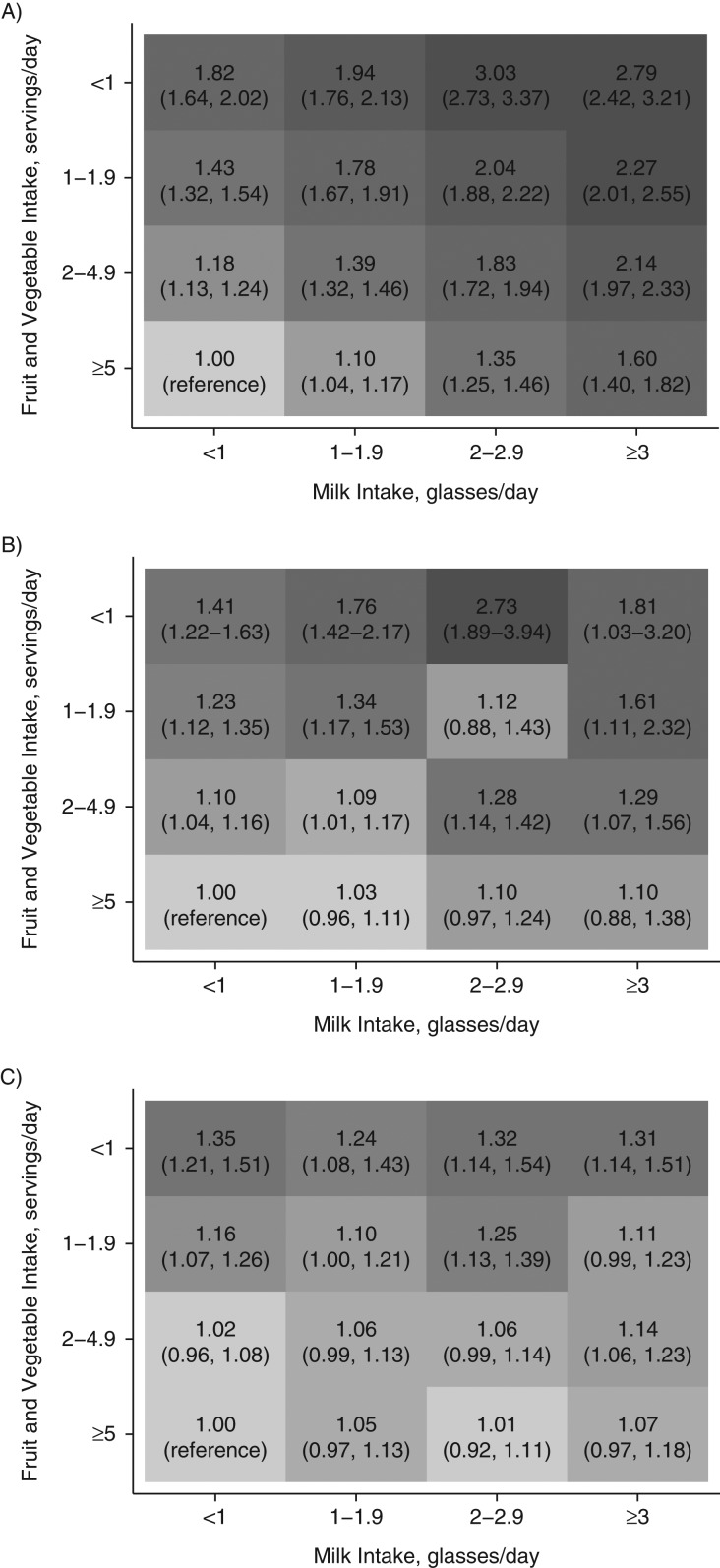
In further analyses, we combined milk intake with fruit and vegetable consumption, as well as with ORACs. In Table 4, we present age-adjusted rates and numbers of deaths by each combination category. The rate of mortality was highest among persons consuming less than 1 serving of fruit and vegetables per day (or in the lowest quartile of ORAC) combined with a high consumption of milk, in both men and women. In contrast, the lowest age-adjusted mortality rates were found in women and men who reported high consumption of fruits and vegetables or had high ORACs combined with low intake of milk.
Table 4.
Age-Standardized Rates of Mortality in the Swedish Mammography Cohort (Women) and the Cohort of Swedish Men (Men) per 1,000 Person-Years at Risk, According to Milk Consumption, Fruit and Vegetable Intake, and Quartile of Oxygen Radical Absorbance Capacity, 1987–2015a
| Milk Intake, glasses/day | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–<2 | 2–<3 | ≥3 | |||||||||||||||||
| Mortality Rate | 95% CI | No. of Cases | PY at Risk | Total No.b | Mortality Rate | 95% CI | No. of Cases | PY at Risk | Total No. | Mortality Rate | 95% CI | No. of Cases | PY at Risk | Total No. | Mortality Rate | 95% CI | No. of Cases | PY at Risk | Total No. | |
| Swedish Mammography Cohort (Women), Baseline 1987–1990 (Time-Updated Analysesc) | ||||||||||||||||||||
| Fruit/vegetable intake, servings/day | ||||||||||||||||||||
| <1 | 22.5 | 20.3, 24.8 | 416 | 16,627 | 23.3 | 21.3, 25.4 | 509 | 20,458 | 28.6 | 25.8, 31.7 | 423 | 15,336 | 27.1 | 23.3, 31.5 | 213 | 8,424 | ||||
| 1–<2 | 17.1 | 16.1, 18.2 | 971 | 53,107 | 19.6 | 18.5, 20.7 | 1,264 | 62,158 | 19.8 | 18.3, 21.3 | 776 | 42,498 | 21.5 | 19.1, 24.2 | 310 | 17,065 | ||||
| 2–<5 | 14.1 | 13.7, 14.5 | 4,116 | 283,776 | 15.5 | 15.0, 15.9 | 4,389 | 282,627 | 17.6 | 16.9, 18.4 | 2,257 | 143,057 | 19.4 | 17.9, 21.0 | 734 | 47,815 | ||||
| ≥5 | 12.1 | 11.7, 12.6 | 2,786 | 218,644 | 13.3 | 12.8, 13.9 | 2,168 | 149,108 | 15.1 | 14.1, 16.2 | 812 | 56,873 | 17.1 | 15.0, 19.4 | 247 | 16,597 | ||||
| Swedish Mammography Cohort (Women), Baseline 1997 | ||||||||||||||||||||
| Fruit/vegetable intake, servings/day | ||||||||||||||||||||
| <1 | 27.7 | 23.8, 32.1 | 219 | 413 | 32.5 | 25.5, 40.7 | 92 | 160 | 41.8 | 28.0, 60.0 | 29 | 52 | 40.2 | 19.9, 72.0 | 12 | 20 | ||||
| 1–<2 | 21.9 | 20.1, 23.7 | 624 | 1,541 | 23.8 | 20.8, 27.2 | 240 | 528 | 21.6 | 16.6, 27.6 | 66 | 171 | 29.3 | 18.9, 43.6 | 29 | 51 | ||||
| 2–<5 | 18.2 | 17.6, 18.8 | 3,277 | 11,827 | 18.8 | 17.7, 20.0 | 1,124 | 3,325 | 21.5 | 19.3, 23.8 | 373 | 1,025 | 23.6 | 19.4, 28.4 | 113 | 296 | ||||
| ≥5 | 16.0 | 15.4, 16.7 | 2,827 | 12,836 | 17.4 | 16.3, 18.6 | 926 | 3,269 | 18.8 | 16.7, 21.2 | 284 | 909 | 18.0 | 14.2, 22.5 | 79 | 291 | ||||
| Cohort of Swedish Men, Baseline 1997 | ||||||||||||||||||||
| Fruit/vegetable intake, servings/day | ||||||||||||||||||||
| <1 | 32.8 | 29.6, 36.3 | 383 | 774 | 32.2 | 27.9, 37.1 | 227 | 399 | 32.6 | 27.8, 38.0 | 191 | 331 | 32.5 | 28.4, 37.1 | 228 | 457 | ||||
| 1–<2 | 25.7 | 24.1, 27.5 | 890 | 2,354 | 26.4 | 24.2, 28.7 | 571 | 1,300 | 28.3 | 25.8, 30.9 | 488 | 1,097 | 26.5 | 24.1, 29.2 | 438 | 1,133 | ||||
| 2–<5 | 21.4 | 20.7, 22.2 | 3,291 | 10,889 | 22.4 | 21.4, 23.4 | 2,087 | 5,979 | 23.2 | 22.0, 24.4 | 1,434 | 4,100 | 24.6 | 23.3, 26.0 | 1,312 | 3,773 | ||||
| ≥5 | 20.2 | 19.3, 21.2 | 1,754 | 6,127 | 21.7 | 20.4, 23.0 | 1,030 | 3,125 | 21.4 | 19.7, 23.1 | 634 | 1,931 | 22.9 | 21.0, 24.9 | 520 | 1,511 | ||||
| Swedish Mammography Cohort (Women), Baseline 1997d | ||||||||||||||||||||
| ORAC quartile | ||||||||||||||||||||
| 1 | 20.0 | 19.2, 20.8 | 2,298 | 6,850 | 22.2 | 20.6, 24.0 | 698 | 1,705 | 25.9 | 22.4, 29.8 | 202 | 467 | 27.0 | 21.0, 34.1 | 76 | 157 | ||||
| 2 | 17.3 | 16.4, 18.1 | 1,711 | 6,729 | 19.0 | 17.5, 20.6 | 586 | 1,759 | 21.1 | 18.2, 24.3 | 194 | 555 | 22.1 | 15.9, 29.8 | 43 | 135 | ||||
| 3 | 16.9 | 16.0, 17.7 | 1,622 | 6,667 | 17.0 | 15.6, 18.5 | 538 | 1,843 | 19.0 | 16.1, 22.2 | 159 | 508 | 19.7 | 14.6, 25.9 | 51 | 161 | ||||
| 4 | 15.8 | 15.0, 16.7 | 1,316 | 6,371 | 17.3 | 15.9, 18.8 | 560 | 1,975 | 18.5 | 16.0, 21.2 | 197 | 627 | 19.5 | 15.0, 25.0 | 63 | 205 | ||||
| Cohort of Swedish Men, Baseline 1997e | ||||||||||||||||||||
| ORAC quartile | ||||||||||||||||||||
| 1 | 25.1 | 24.1, 26.2 | 2,180 | 5,517 | 26.7 | 25.1, 28.3 | 1,160 | 2,564 | 28.1 | 26.2, 30.1 | 833 | 1,731 | 29.7 | 27.4, 32.0 | 659 | 1,508 | ||||
| 2 | 21.8 | 20.7, 22.9 | 1,617 | 5,154 | 21.6 | 20.3, 23.1 | 943 | 2,645 | 24.1 | 22.4, 25.9 | 710 | 1,857 | 25.4 | 23.4, 27.4 | 625 | 1,664 | ||||
| 3 | 20.3 | 19.2, 21.4 | 1,361 | 4,906 | 22.2 | 20.8, 23.6 | 947 | 2,809 | 22.3 | 20.6, 24.2 | 610 | 1,904 | 23.2 | 21.3, 25.2 | 566 | 1,701 | ||||
| 4 | 19.9 | 18.7, 21.1 | 1,160 | 4,567 | 21.6 | 20.2, 23.1 | 865 | 2,785 | 20.9 | 19.2, 22.7 | 594 | 1,967 | 23.1 | 21.3, 24.9 | 648 | 2,001 | ||||
Abbreviations: CI, confidence interval; ORAC, oxygen radical absorbance capacity; PY, person-years.
a Baseline dates varied by cohort and analysis. Follow-up ended on September 30, 2015.
b Total number of participants in category.
c Because of the time-updated exposures, person-years at risk are given.
d Median ORAC value: quartile 1 (<9,546 µmol/day), 7,914 µmol/day; quartile 2 (9,546–<12,217 µmol/day), 10,892 µmol/day; quartile 3 (12,217–<15,488 µmol/day), 13,653 µmol/day; quartile 4 (≥15,488 µmol/day), 18,331 µmol/day.
e Median ORAC value: quartile 1 (<10,880 µmol/day), 8,967 µmol/day; quartile 2 (10,880–<13,881 µmol/day), 12,418 µmol/day; quartile 3 (13,881–<17,457 µmol/day), 15,477 µmol/day; quartile 4 (≥17,457 µmol/day), 20,490 µmol/day.
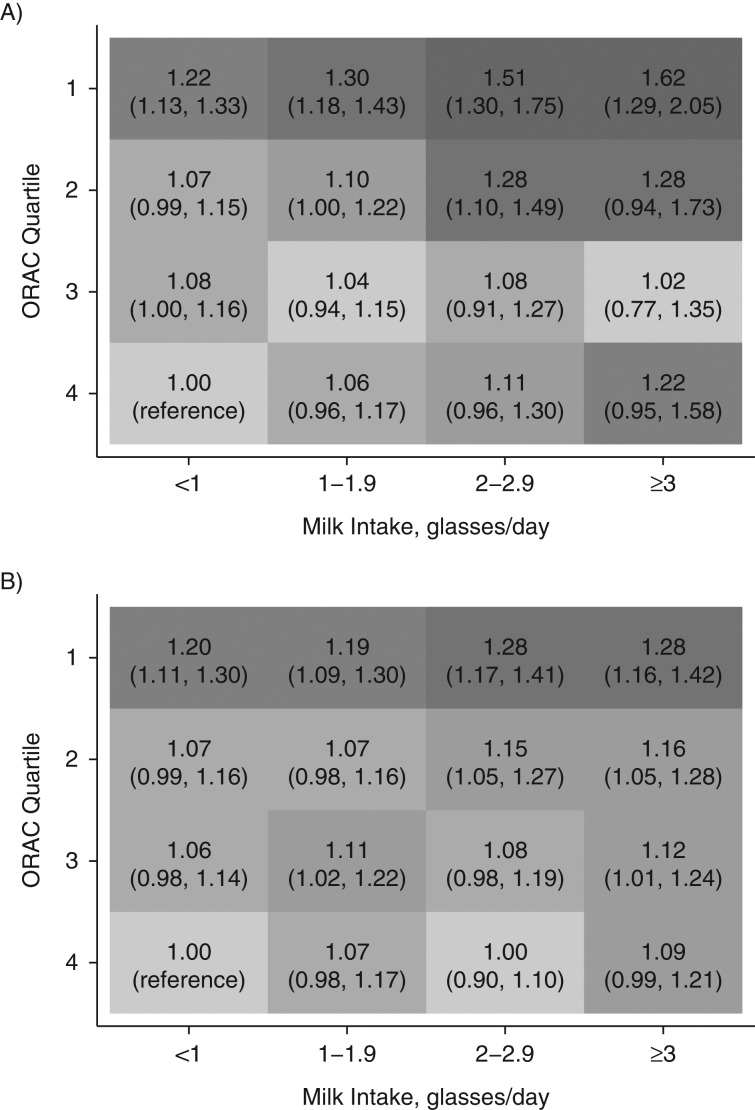
Figures 2 and 3 depict the multivariable-adjusted hazard ratios for mortality by milk and fruit/vegetable intake or ORAC, using the group with the lowest intake of milk (<1 glass/day) and the highest intake of fruit and vegetables (≥5 servings/day) or ORAC (highest quartile) as the reference. Hazard ratios for mortality tended to increase with higher milk consumption in every category of fruit and vegetable intake or ORAC, although the estimates were attenuated with increasing consumption of fruits and vegetables or ORAC. The pattern was clearer with time-updated information as compared with a single exposure assessment. Accordingly, in time-updated analysis of the SMC, a high intake of milk (≥3 glasses/day) with a concomitant low intake of fruit and vegetables (<1 serving/day) conferred a multivariable-adjusted hazard ratio of 2.79 (95% confidence interval (CI): 2.42, 3.21), and with a combined high intake of fruit and vegetables, the hazard ratio was 1.60 (95% CI: 1.40, 1.82). In women with a single exposure assessment, the corresponding estimates were 1.81 (95% CI: 1.03, 3.20) and 1.10 (95% CI: 0.88, 1.38), respectively. The same comparisons in men revealed a hazard ratio of 1.31 (95% CI: 1.14, 1.51) for high consumers of milk with a low fruit and vegetable intake and a hazard ratio of 1.07 (95% CI: 0.97, 1.18) for high consumers of milk who also consumed 5 or more servings of fruits and vegetables per day. If we used ORAC intake instead of fruit and vegetable intake as the effect-measure modifier, the estimates remained essentially unaltered. The relative excess risk of interaction estimate of 0.37 (95% CI: 0.01, 1.27) in the time-updated analysis of women indicated a modest additive interaction. No significant interaction was discovered among men (data not shown).
Figure 2.
Adjusted hazard ratios (HRs) and 95% confidence intervals (in parentheses) for all-cause mortality according to combined intakes of milk and fruit and vegetables, using persons with the lowest milk intake and the highest fruit and vegetable intake as the reference group. A) HRs based on time-updated information on the whole Swedish Mammography Cohort (SMC) (women, baseline 1987–1990); B) HRs based on the SMC after administration of the second food frequency questionnaire (women, baseline 1997); C) HRs based on the Cohort of Swedish Men (men, baseline 1997). The shading corresponds to the value of the HR; the darker the shading, the larger the HR. One glass of milk corresponds to 200 mL. Covariates were age, body mass index (weight (kg)/height (m)2), height, energy intake, alcohol intake, intakes of yogurt, cheese, and red and processed meat, education, marital status (living alone vs. not), physical activity (metabolic equivalent-hours/day), smoking habits (never, former, or current smoker and, for baseline 1997, also pack-years of smoking), ever use of antioxidant-containing supplements, and weighted Charlson's comorbidity index.
Figure 3.
Adjusted hazard ratios (HRs) and 95% confidence intervals (in parentheses) for all-cause mortality according to combined daily intake of milk and oxygen radical absorbance capacity (ORAC; µmol/day), using persons with the lowest intake of milk and the highest quartile of ORAC as the reference group. A) HRs based on the Swedish Mammography Cohort after administration of the second food frequency questionnaire (women, baseline 1997); B) HRs based on the Cohort of Swedish Men (men, baseline 1997). The shading corresponds to the value of the HR; the darker the shading, the larger the HR. One glass of milk corresponds to 200 mL. Covariates were age, body mass index (weight (kg)/height (m)2), height, energy intake, alcohol intake, intakes of yogurt, cheese, and red and processed meat, education, marital status (living alone vs. not), physical activity (metabolic equivalent-hours/day), smoking habits (never, former, or current smoker and pack-years of smoking), ever use of antioxidant-containing supplements, and weighted Charlson's comorbidity index.
Hazard ratios were not attenuated in women after additional adjustment, including adjustment for vitamin and mineral nutrients common in milk, although they were somewhat attenuated in men (see Web Table 1, available at http://aje.oxfordjournals.org/). The total number of cardiovascular disease or cancer deaths was less than half that of the number of deaths from any cause (Web Tables 2 and 3). Nevertheless, for cardiovascular mortality (Web Table 4), the hazard ratios remained similar to estimates of all-cause mortality, whereas hazard ratios for cancer mortality were lower (Web Table 5). Exclusion of the first 2 years of follow-up (Web Table 6; 2%–5% of all deaths were excluded, depending on the analysis), persons with a body mass index greater than 35 (Web Table 7; 2% of all deaths were excluded), and current smokers (Web Table 8; 24%–29% of all deaths were excluded) gave estimates similar to those seen in the total cohort.
DISCUSSION
In 2 independent population-based cohorts, mortality rates were highest in persons with high consumption of milk combined with low consumption of fruits and vegetables or a low ORAC. However, the gradient of risk with increasing milk consumption was more pronounced in women, and an additive interaction for mortality rates between milk consumption and fruit and vegetable consumption was found only in women.
The findings of our observational investigation should not be evaluated in isolation and have to be interpreted cautiously. A recent attempt to perform a meta-analysis demonstrated substantial heterogeneity in nonfermented milk consumption among cohort studies in relation to mortality from all causes (43). Heterogeneity among studies was observed in most subgroups defined by sex, country, and study quality (43). Besides methodological differences, a potential explanation for the inconsistent findings may be related to the variability in the range of milk consumption in different populations and, as also indicated by our present study, by different patterns of intake of antioxidant-rich foods in the populations. To our knowledge, no randomized trial has examined the association of milk intake with incidence of mortality, and this study design is unlikely to ever be implemented. Another possible analytical approach might be the use of genetic variation in lactase persistence within a Mendelian randomization study design, but this specific genetic variant is probably weak as an instrumental variable (44), with conceivable pleiotropic effects (45, 46).
The present study extends our previous finding of higher mortality rates with high milk consumption (1). Our postulated mechanism is that milk consumption induces oxidative stress by way of the galactose component of lactose, because galactose supplementation results in premature aging in animals through induction of oxidative stress and inflammation (2–5). Oxidative stress induced by galactose is a consequence of an imbalance between prooxidant and antioxidant defenses, which causes accumulation of advanced glycation end-products and reactive oxygen species, especially superoxide radicals and hydrogen peroxide (2–5). Indeed, we have previously noted higher concentrations of oxidative stress and inflammation markers in human urine and serum with high consumption of milk (1).
Mortality rates were increased at more moderate levels of milk consumption in women as compared with men; excess mortality was seen with 1–2 glasses of milk per day in women, while twice that amount was necessary for the observation of excess mortality in men. A sex difference in sensitivity to galactose exposure has been identified experimentally (6–8), and galactose elimination capacity is also higher in men than in women, but it declines with increasing age (47–49).
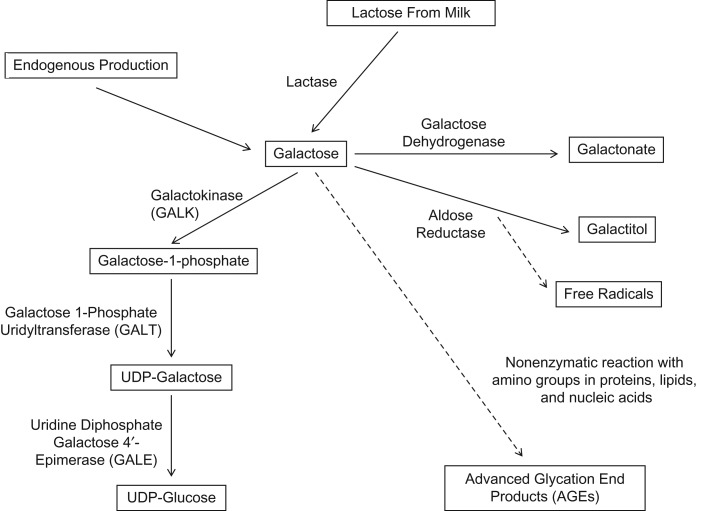
Galactose is used in the endogenous production of human breast milk. Most lactose in human breast milk is synthesized from galactose taken up from the blood, and only one-third is made from endogenous synthesis (50). Liver glycogen in infants is formed mainly from breast milk–derived galactose (51), and it acts as a reservoir for subsequent hepatic glucose release to the circulation during times of fasting (52, 53). A lower female degradation of galactose might have been an evolutionary survival mechanism for the child. Specifically, the enzyme galactose-1-phosphate uridylyltransferase (GALT) in the Leloir breakdown pathway of galactose (Figure 4) has a higher activity in male animals than in female animals (7, 54, 55). GALT deficiency is also the main cause of galactosemia, an inborn error of metabolism resulting in early death without avoidance of galactose intake. With lower capacity of galactose degradation to glucose by the Leloir pathway, an alternative route is the polyol pathway, where galactose is converted to galactitol by aldose reductase, secondarily leading to free-radical formation (56, 57). In addition, galactose reacts nonenzymatically with amino groups in proteins and peptides, forming advanced glycation end-products (3). Although the exact mechanisms are not known, galactose-treated rodents, flies, and tissue culture cells also display evidence of lower-than-expected antioxidant enzyme activities, suggesting that the normal defenses might be compromised (2, 5, 58).
Figure 4.
Overview of galactose metabolism. The major pathway of galactose metabolism (the Leloir pathway) operates via the enzymes galactokinase (GALK), galactose-1-phosphate uridylyltransferase (GALT), and uridine diphosphate (UDP) galactose 4-epimerase (GALE), resulting in UDP-glucose. The conversion to galactitol by aldose reductase via the polyol pathway results in decreased availability of nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione, with increased production of free radicals (56). By way of a nonenzymatic reaction with amino groups in proteins, lipids, and nucleic acids, galactose is converted to advanced glycation end products (AGEs).
By reducing oxidative stress and inflammation processes, higher fruit and vegetable intake has convincingly been shown to promote longevity (17, 59) and reduce the risk of cardiovascular disease (13, 17, 60) and some cancers (61). Intriguingly, and supporting our results in women, there is experimental evidence that fruits and vegetables or extracts of them can rescue animals from the premature aging phenotype induced by galactose supplementation (20–24, 62, 63).
We found no clear interaction between milk intake and fruit and vegetable intake in men. This failure to find an interaction could have several explanations. The association between milk and mortality was more modest in men, and a clear excess mortality rate was observed in men only above ≥3 glasses/day, which limited our possibility to detect an interaction pattern with fruit and vegetables. Furthermore, the gene expression and activity of antioxidant enzymes (such as mitochondrial glutathione peroxidase and superoxide dismutase) in animals seem to be higher in females than in males, giving females an enhanced capacity to provide mitigation of oxidative damage through an increased intake of antioxidants (64).
The main strengths of this study were the use of data from 2 large population-based cohorts and the comprehensive FFQ administered in a setting with a wide range of milk intakes and intakes of antioxidant foods. We found consistency in the results irrespective of whether fruit and vegetable consumption or ORAC was used as the effect measure modifier. Loss to follow-up was negligible, because we used the individual personal identification number for linkage to the death registry. Through the use of time-updated information and with a larger number of outcomes in the SMC, we observed stronger estimates compared with use of a single assessment of exposure. Questions regarding fruit and vegetable intake were more diversified in the second FFQ, administered in 1997, but still the mortality rate patterns with a threshold at 5 servings/day were similar (Figure 1B). Our results might not apply to people of other ethnic origins, such as those with a high prevalence of lactose intolerance, or to children and adolescents.
Our observational results in this population of Swedish adults question the value of recommending high consumption of milk, especially in women not meeting the recommended requirements for fruit and vegetable intake (≥5 servings/day).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Unit of Orthopedics, Department of Surgical Sciences, Faculty of Medicine, Uppsala University, Uppsala, Sweden (Karl Michaëlsson, Liisa Byberg); Unit of Clinical Pharmacology, Department of Medical Sciences, Faculty of Medicine, Uppsala University, Uppsala, Sweden (Håkan Melhus); and Unit of Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden (Alicja Wolk).
This work was supported by grants from the Swedish Research Council.
The funding organization played no role in the design or conduct of the study.
Conflict of interest: none declared.
REFERENCES
- 1. Michaëlsson K, Wolk A, Langenskiold S, et al. . Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui X, Zuo P, Zhang Q, et al. . Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res. 2006;83(8):1584–1590. [DOI] [PubMed] [Google Scholar]
- 3. Song X, Bao M, Li D, et al. . Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999;108(3):239–251. [DOI] [PubMed] [Google Scholar]
- 4. Hao L, Huang H, Gao J, et al. . The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced by D-galactose in mice. Neurosci Lett. 2014;571C:45–49. [DOI] [PubMed] [Google Scholar]
- 5. Cui X, Wang L, Zuo P, et al. . D-galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology. 2004;5(5):317–325. [DOI] [PubMed] [Google Scholar]
- 6. Lin YN, Radin NS. Sexual differences in galactose metabolism: galactosyl ceramide galactosidase and other galactosidases in mouse kidney. Biochem J. 1973;136(4):1125–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parkhurst GW, Mayes JS. Galactose toxicity and activities of the galactose-metabolizing enzymes during development of the chick. Arch Biochem Biophys. 1972;150(2):742–745. [DOI] [PubMed] [Google Scholar]
- 8. Nordin JH, Wilken DR, Bretthauer RK, et al. . A consideration of galactose toxicity in male and female chicks. Poultry Sci. 1960;39(4):802–812. [Google Scholar]
- 9. Reuter S, Gupta SC, Chaturvedi MM, et al. . Oxidative stress, inflammation, and cancer: how are they linked. Free Radic Biol Med. 2010;49(11):1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michaëlsson K, Wolk A, Byberg L, et al. . Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am J Clin Nutr. 2014;99(1):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30(5):511–517. [DOI] [PubMed] [Google Scholar]
- 13. Estruch R, Ros E, Salas-Salvado J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. [DOI] [PubMed] [Google Scholar]
- 14. Fito M, Guxens M, Corella D, et al. . Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167(11):1195–1203. [DOI] [PubMed] [Google Scholar]
- 15. Kris-Etherton PM, Hu FB, Ros E, et al. . The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138(9):1746S–1751S. [DOI] [PubMed] [Google Scholar]
- 16. Bellavia A, Larsson SC, Bottai M, et al. . Fruit and vegetable consumption and all-cause mortality: a dose-response analysis. Am J Clin Nutr. 2013;98(2):454–459. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Ouyang Y, Liu J, et al. . Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rautiainen S, Serafini M, Morgenstern R, et al. . The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am J Clin Nutr. 2008;87(5):1247–1253. [DOI] [PubMed] [Google Scholar]
- 19. Rautiainen S, Levitan EB, Orsini N, et al. . Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med. 2012;125(10):974–980. [DOI] [PubMed] [Google Scholar]
- 20. Coban J, Dogan-Ekici I, Aydin AF, et al. . Blueberry treatment decreased D-galactose-induced oxidative stress and brain damage in rats. Metab Brain Dis. 2015;30(3):793–802. [DOI] [PubMed] [Google Scholar]
- 21. Coban J, Betul-Kalaz E, Kucukgergin C, et al. . Blueberry treatment attenuates D-galactose-induced oxidative stress and tissue damage in rat liver. Geriatr Gerontol Int. 2014;14(2):490–497. [DOI] [PubMed] [Google Scholar]
- 22. Stefek M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip Toxicol. 2011;4(2):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghanbari S, Yonessi M, Mohammadirad A, et al. . Effects of IMOD™ and Angipars™ on mouse D-galactose-induced model of aging. Daru. 2012;20(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohammadirad A, Aghamohammadali-Sarraf F, Badiei S, et al. . Anti-aging effects of some selected Iranian folk medicinal herbs—biochemical evidences. Iran J Basic Med Sci. 2013;16(11):1170–1180. [PMC free article] [PubMed] [Google Scholar]
- 25. European Food Information Council Fruit and Vegetable Consumption in Europe—Do Europeans Get Enough? Brussels, Belgium:European Food Information Council; 2012. [Google Scholar]
- 26. Boffetta PL, Couto E, Wichmann J, et al. . Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2010;102(8):529–537. [DOI] [PubMed] [Google Scholar]
- 27. Willett W. Nutritional Epidemiology. 3rd ed New York, NY: Oxford University Press; 2013. [Google Scholar]
- 28. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 29. Thomas LD, Michaëlsson K, Julin B, et al. . Dietary cadmium exposure and fracture incidence among men: a population-based prospective cohort study. J Bone Miner Res. 2011;26(7):1601–1608. [DOI] [PubMed] [Google Scholar]
- 30. Warensjö E, Byberg L, Melhus H, et al. . Dietary calcium intake and risk of fracture and osteoporosis: prospective longitudinal cohort study. BMJ. 2011;342:d1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larsson SC, Bergkvist L, Wolk A. Long-term dietary calcium intake and breast cancer risk in a prospective cohort of women. Am J Clin Nutr. 2009;89(1):277–282. [DOI] [PubMed] [Google Scholar]
- 32. Brugård Konde Å, Bjerselius R, Haglund L, et al. . Swedish Dietary Guidelines—Risk and Benefit Management Report (Livsmedelsverkets rapportserie no. 5/2015). Uppsala, Sweden: Livsmedelsverket (National Food Agency); 2015. http://www.livsmedelsverket.se/globalassets/rapporter/2015/rapp-hanteringsrapport-engelska-omslag--inlaga--bilagor-eng-version.pdf. Accessed April 21, 2016.
- 33. Hansson LM, Galanti MR. Diet-associated risks of disease and self-reported food consumption: how shall we treat partial nonresponse in a food frequency questionnaire. Nutr Cancer. 2000;36(1):1–6. [DOI] [PubMed] [Google Scholar]
- 34. Bergstrom L, Kylberg E, Hagman U, et al. . The food composition database KOST: the National Administration's information system for nutritive values of food [in Swedish]. Vår Föda. 1991;43:439–447. [Google Scholar]
- 35. Larsson SC, Andersson SO, Johansson JE, et al. . Cultured milk, yoghurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am J Clin Nutr. 2008;88(4):1083–1087. [DOI] [PubMed] [Google Scholar]
- 36. Natella F, Nardini M, Giannetti I, et al. . Coffee drinking influences plasma antioxidant capacity in humans. J Agric Food Chem. 2002;50(21):6211–6216. [DOI] [PubMed] [Google Scholar]
- 37. StataCorp LP Stata Reference Manual, Release 11. College Station, TX: Stata Press; 2009. [Google Scholar]
- 38. VanderWeele TJ, Hernan MA, Robins JM. Causal directed acyclic graphs and the direction of unmeasured confounding bias. Epidemiology. 2008;19(5):720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 40. Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 41. Horton NJ, Kleinman KP. Much ado about nothing: a comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larsson SC, Crippa A, Orsini N, et al. . Non-fermented milk consumption and mortality from all causes, cardiovascular disease, and cancer: a systematic review and meta-analysis. Nutrients. 2015;7(9):7749–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Timpson NJ, Brennan P, Gaborieau V, et al. . Can lactase persistence genotype be used to reassess the relationship between renal cell carcinoma and milk drinking? Potentials and problems in the application of Mendelian randomization. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corella D, Arregui M, Coltell O, et al. . Association of the LCT-13910C>T polymorphism with obesity and its modulation by dairy products in a Mediterranean population. Obesity. 2011;19(8):1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wagh K, Bhatia A, Alexe G, et al. . Lactase persistence and lipid pathway selection in the Maasai. PLoS One. 2012;7(9):e44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tygstrup N. The galactose elimination capacity in control subjects and in patients with cirrhosis of the liver. Acta Med Scand. 1964;175:281–289. [DOI] [PubMed] [Google Scholar]
- 48. Schnegg M, Lauterburg BH. Quantitative liver function in the elderly assessed by galactose elimination capacity, aminopyrine demethylation and caffeine clearance. J Hepatol. 1986;3(2):164–171. [DOI] [PubMed] [Google Scholar]
- 49. Marchesini G, Bua V, Brunori A, et al. . Galactose elimination capacity and liver volume in aging man. Hepatology. 1988;8(5):1079–1083. [DOI] [PubMed] [Google Scholar]
- 50. Sunehag A, Tigas S, Haymond MW. Contribution of plasma galactose and glucose to milk lactose synthesis during galactose ingestion. J Clin Endocrinol Metab. 2003;88(1):225–229. [DOI] [PubMed] [Google Scholar]
- 51. Kunst C, Kliegman R, Trindade C. The glucose-galactose paradox in neonatal murine hepatic glycogen synthesis. Am J Physiol. 1989;257(5):E697–E703. [DOI] [PubMed] [Google Scholar]
- 52. Brown LD, Cavalli C, Harwood JE, et al. . Plasma concentrations of carbohydrates and sugar alcohols in term newborns after milk feeding. Pediatr Res. 2008;64(2):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spedale SB, Battaglia FC, Sparks JW. Hepatic metabolism of glucose, galactose, and lactate after milk feeding in newborn lambs. Am J Physiol. 1992;262(1):E46–E51. [DOI] [PubMed] [Google Scholar]
- 54. Mayes JS, Miller LR, Myers FK. The relationship of galactose-1-phosphate accumulation and uridyl transferase activity to the differential galactose toxicity in male and female chicks. Biochem Biophys Res Commun. 1970;39(4):661–665. [DOI] [PubMed] [Google Scholar]
- 55. McCluer RH, Gross SK. Biosynthesis of neutral glycosphingolipids in kidney slices from male and female mice. J Lipid Res. 1985;26(5):593–599. [PubMed] [Google Scholar]
- 56. Kubo E, Miyoshi N, Fukuda M, et al. . Cataract formation through the polyol pathway is associated with free radical production. Exp Eye Res. 1999;68(4):457–464. [DOI] [PubMed] [Google Scholar]
- 57. Lai K, Elsas LJ, Wierenga KJ. Galactose toxicity in animals. IUBMB Life. 2009;61(11):1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jumbo-Lucioni PP, Hopson ML, Hang D, et al. . Oxidative stress contributes to outcome severity in a Drosophila melanogaster model of classic galactosemia. Dis Model Mech. 2013;6(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang X, Shu XO, Xiang YB, et al. . Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr. 2011;94(1):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367(9507):320–326. [DOI] [PubMed] [Google Scholar]
- 61. Giacosa A, Barale R, Bavaresco L, et al. . Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev. 2013;22(1):90–95. [DOI] [PubMed] [Google Scholar]
- 62. Chang L, Liu X, Liu J, et al. . D-galactose induces a mitochondrial complex I deficiency in mouse skeletal muscle: potential benefits of nutrient combination in ameliorating muscle impairment. J Med Food. 2014;17(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lu J, Wu DM, Zheng YL, et al. . Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kappaB pathway activation. Cereb Cortex. 2010;20(11):2540–2548. [DOI] [PubMed] [Google Scholar]
- 64. Borras C, Sastre J, Garcia-Sala D, et al. . Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34(5):546–552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.