Figure 5.
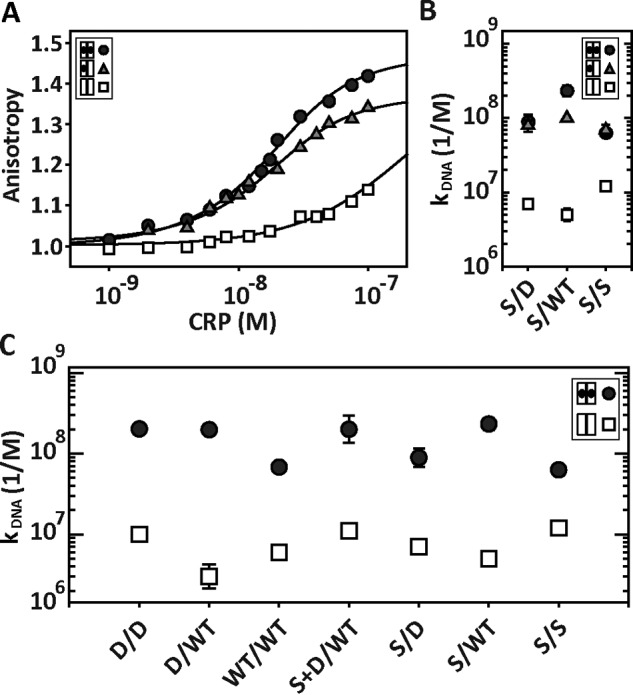
DNA-CRPSC interactions using saturating and non-saturating cAMP concentrations. A, interaction of CRPSCS/D with the 32-bp lac promoter using 0, 30, and 1000 μm cAMP, which correspond to unbound (open squares), singly (gray triangles), and doubly (black circles) cAMP-bound states. The solid lines represent the fit as described in Ref. 32. B, DNA binding affinity constants of CRPSCS/D, CRPSCS/WT, and CRPSCS/S when the proteins are in the unbound (open squares), singly (gray triangles), and doubly (black circles) cAMP-bound states. C, DNA binding affinity constants obtained from fluorescence anisotropy experiments for symmetric and asymmetric CRPSC in the absence (open squares) and presence of saturating cAMP concentrations (black circles). For CRPSCWT/WT, CRPSCD/D, and CRPSCD/WT, [cAMP] = 200 μm. For CRPSCS/S, [cAMP] = 2000 μm. For CRPSCS/D, CRPSCS/WT, and CRPSCS+D/WT, [cAMP] = 1000 μm.
