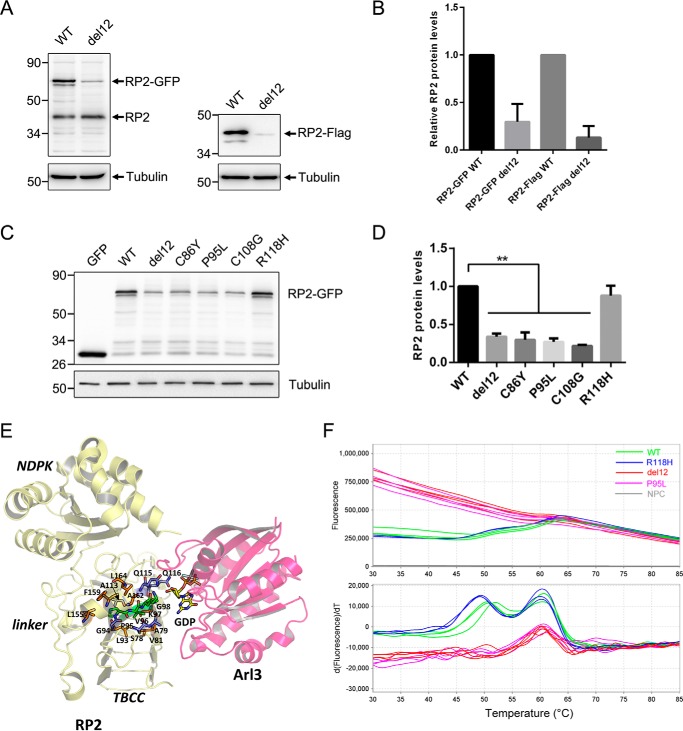
Figure 3.
The del12 and nearby mutations in human RP2 decreased its protein level and stability. A, C-terminal GFP or FLAG-tagged WT and del12 mutant RP2 were transfected into ARPE-19 cells. The RP2 protein levels were detected by Western blot using the anti-human RP2 antibody or the anti-FLAG antibody. The endogenous and GFP or FLAG-tagged RP2 bands are indicated by arrows. Tubulin was used as a loading control. B, the quantitative results of A from five independent experiments are shown as the mean with S.D. (n = 5). The del12 mutation significantly reduced the protein levels of RP2. C, the nearby C86Y, P95L, and C108G mutations of RP2 show similar effects as the del12 mutation on the expression of RP2 in ARPE-19 cells. The R118H mutation was used as a control. GFP, the empty vector without RP2 sequence. D, the quantitative results of C from three independent experiments are shown as the mean with S.D. (n = 3). **, p < 0.01. E, intraprotein interactions of the four residues affected by the RP2 del12 mutation. Pro-95, Val-96, Lys-97, and Gly-98 are shown in stick representation colored green. Those residues that participate in H-bonds with the four residues are shown in blue, and hydrophobic interactions are in orange. F, the melting curve plots and derivative plots of WT, del12, P95L, and R118H forms of GST-RP2 fusion proteins in DSF analysis are shown. The high initial fluorescence signals of del12 and P95L groups (upper panel, red and purple lines) indicate that the proteins are partially or completely unfolded at the beginning. The two transitions (around 50 and 60 °C) of WT and R118H groups (lower panel, green and blue lines) represent the unfolding processes of RP2 and GST with increasing temperature, respectively. NPC, no protein control.