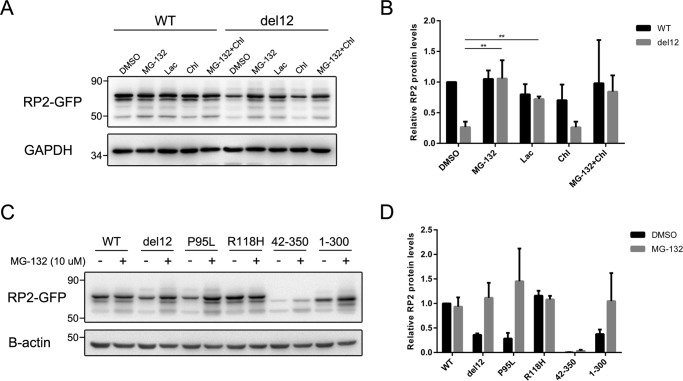
Figure 7.
The unstable RP2 proteins were degraded through proteasome pathway in ARPE-19 cells. A, ARPE-19 cells transfected with WT and del12 mutant RP2 were treated with MG-132 (10 μm), lactacystin (Lac, 20 μm), or chloroquine (Chl, 20 μm) for 18 h. The RP2 protein levels were detected by Western blot. GAPDH was used as a loading control. The experiment shown was repeated at least three times. B, quantitative analysis of the Western blot data shown in A revealed that the proteasome inhibitors MG-132 and lactacystin significantly increased the protein levels of del12 mutant RP2. The results are shown as the mean with S.D. n = 3. **, p < 0.01. C, the P95L, 42–350, and 1–300 forms of RP2 were transfected into ARPE-19 cells, which were further treated with MG-132 (10 μm) for 18 h. RP2 protein levels were detected by Western blot. GAPDH was used as a loading control. The experiment shown was repeated at least three times. D, quantitative results of C from three independent experiments are shown as the mean with S.D. (n = 3). MG-132 treatment markedly increased the protein levels of P95L and 1–300 forms of RP2 mutants.