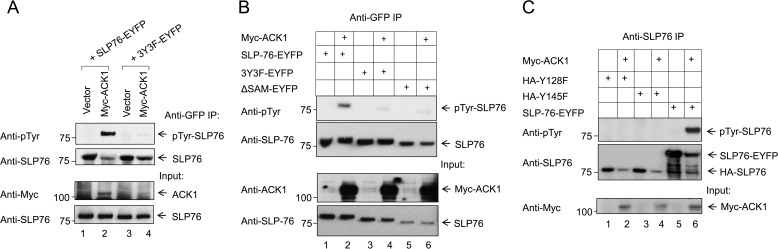
Figure 4.
ACK1 mediates phosphorylation of SLP-76 3Y proximal tyrosines. A, phosphorylation of SLP-76 by ACK1 was assessed by co-transfecting HEK293T cells with ACK1 and WT SLP76-EYFP (lane 2) or tyrosine mutant SLP-76 (3Y3F-EYFP, lane 4) for 24 h, followed by SLP-76 immunoprecipitation (IP) with anti-GFP, and total tyrosine phosphorylation was assessed by 4G10 (anti-Tyr(P)) antibody (first blot). SLP-76 immunoprecipitation efficiency was assessed by anti-SLP76 blotting (second blot), whereas transfection efficiency was assessed by blotting of lysates with anti-Myc (for ACK1) and anti-SLP-76 (for WT and 3Y3F SLP-76). B, ACK1-mediated phosphorylation requires SLP-76 to possess an intact N terminus, as shown by loss of phosphorylation in SLP76-ΔSAM (lane 6) and 3Y3F (lane 4) SLP-76 mutants. WT SLP-76 (lane 2) was efficiently tyrosine-phosphorylated. No detectable phosphorylation was seen in control cells co-transfected with empty Myc-tagged vector (lanes 1, 3, and 5). C, point mutation of Tyr-128 or Tyr-145 abrogated tyrosine phosphorylation of unmutated Tyr-113 and Tyr-145 (lane 2) or Tyr-113 and 128 (lane 4), respectively. Co-transfection of SLP-76 or its mutants with empty vector (lanes 1, 3, and 5) served as a negative control. All blots shown are representative of at least three to four independent experiments. For comparison purposes, the data for Fig. 4, B and C, are derived using the same input lysates as used in Fig. 2, A and B, respectively.