Figure 4.
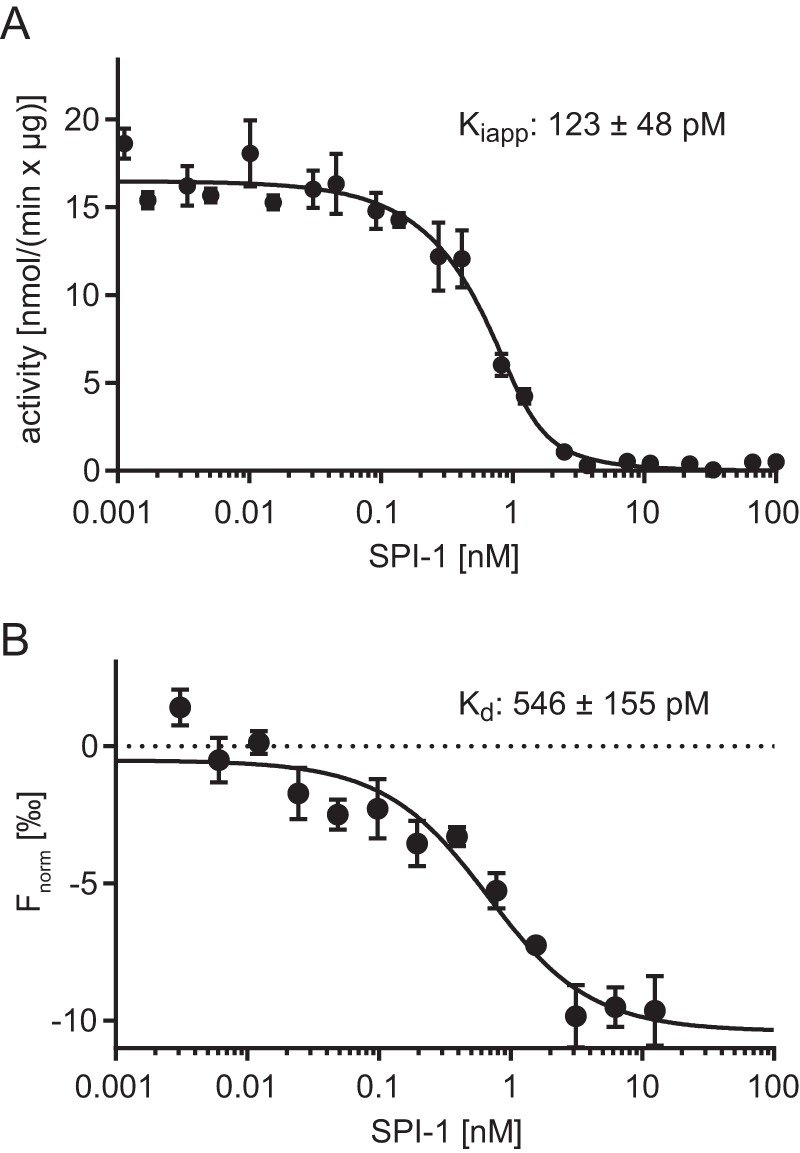
Interaction of SBT4.13 with SPI-1. A, apparent inhibition constant. Purified SBT4.13 (1.5 nm) was incubated with two serial dilutions (1:2) of SPI-1 starting at 100 and 200 nm, respectively. Activity was recorded over 10 min at room temperature using a fluorigenic peptide substrate at 25 μm. An apparent inhibition constant (Ki(app)) of 123 ± 48 pm was calculated using the Morrison equation (39, 40) in GraphPad Prism 6 (R2 = 0.979). Results represent the mean of three biological replicates with independent SPI-1 preparations. B, dissociation constant analyzed by MST. Labeled SBT4.13 (250 pm) was titrated against a serial dilution (1:1) of unlabeled SPI-1 starting at 12.5 nm. The baseline-corrected normalized fluorescence (Fnorm) is plotted against the SPI-1 concentration. A Kd of 546 ± 155 pm was calculated with GraphPad Prism 6 (R2 = 0.911). Results represent the mean ± S.E. (error bars) of three biological replicates using independent SPI-1 preparations, all run in technical triplicates.
