Figure 5.
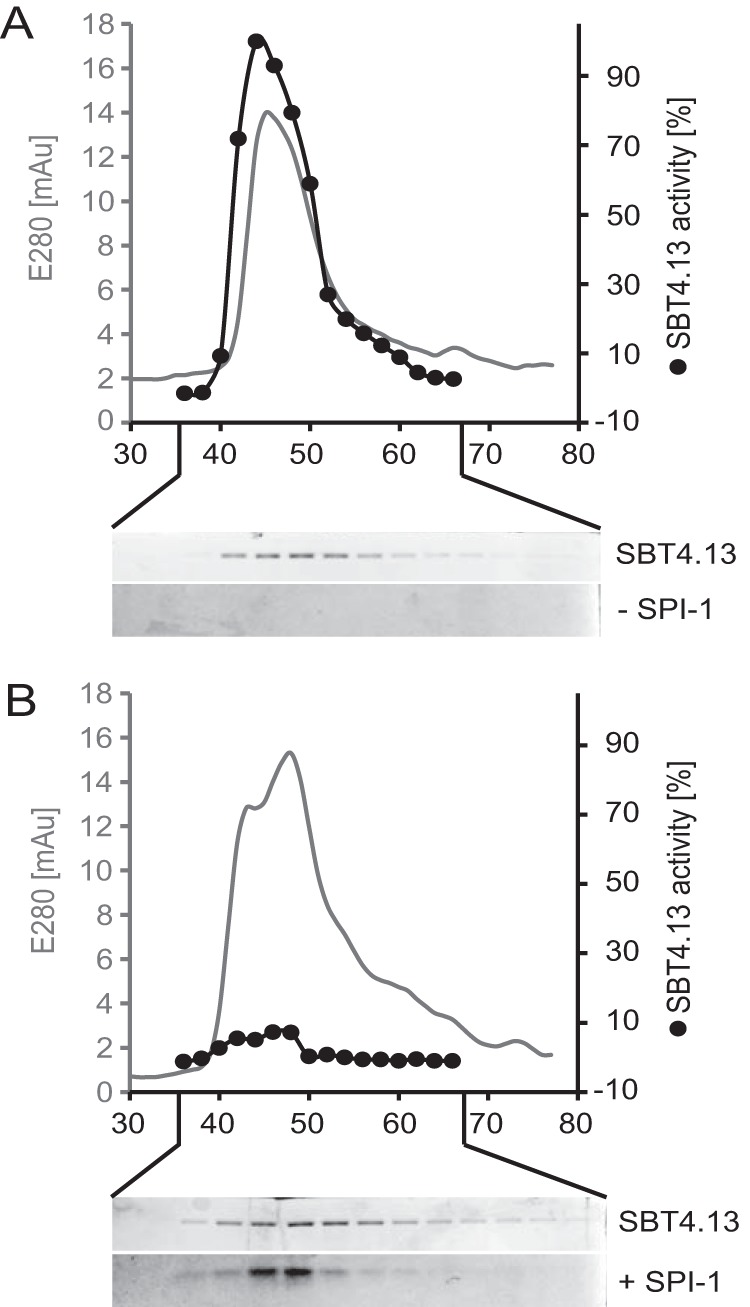
Purification of the SBT4.13·SPI-1 complex. SBT4.13 and the SBT4.13·SPI-1 complex were separated by gel filtration. Fractions were analyzed by SDS-PAGE, and SBT4.13 activity was assayed using a fluorigenic peptide substrate at 25 μm. A, gel filtration of SBT4.13 (100 μg) monitored at 280 nm (gray line) and collected in 200-μl fractions. SBT4.13 activity was assayed in 0.5-μl aliquots (black dots) in two technical replicates. Fifteen-microliter aliquots of the same fractions were separated by 15% SDS-PAGE and Coomassie-stained as shown below the chromatogram. B, SBT4.13 (100 μg) incubated for 10 min in the presence of a 3-fold molar excess of SPI-1 was separated by gel filtration, and fractions were analyzed as described for A. mAU, milliabsorbance units.
