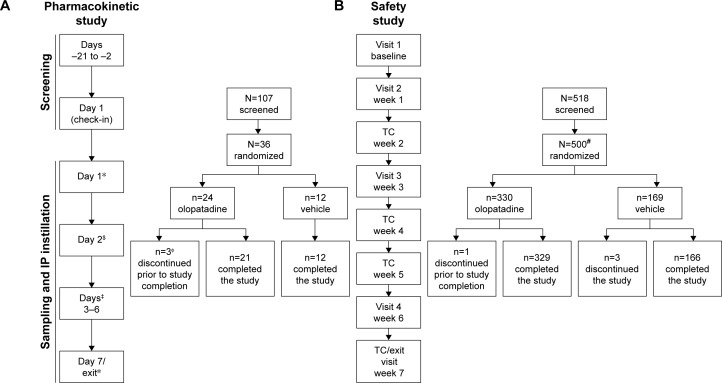
Figure 1.
Study design of (A) pharmacokinetic study and (B) safety study.
Notes: *PK sampling: 0 hour (pre-dose), 0.25, 0.5, 1, 2, 4, 8, and 12 hours. $PK sampling: 0 hour (trough–24 hours after 1st dose-trough). ‡PK sampling: 0 hour (trough). ΦAll 3 subjects withdrew from the study with consent, and the withdrawal was not related to the treatment. #One subject was not included in the safety population because of randomization error and for not receiving the investigational product.
Abbreviations: IP, investigational product; PK, pharmacokinetics; TC, telephonic contact.