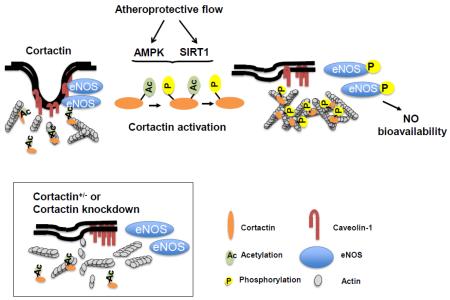
Abstract
Objective
Cortactin translocates to the cell periphery in vascular endothelial cells (ECs) upon cortical actin assembly in response to pulsatile shear stress. Because cortactin has putative sites for AMP-activated protein kinase (AMPK) phosphorylation and sirtuin 1 (SIRT1) deacetylation, we examined the hypothesis that AMPK and SIRT1 coregulate cortactin dynamics in response to shear stress.
Approach and Results
Analysis of the ability of AMPK to phosphorylate recombinant cortactin and oligopeptides whose sequences matched AMPK consensus sequences in cortactin pointed to Thr-401 as the site of AMPK phosphorylation. Mass spectrometry confirmed Thr-401 as the site of AMPK phosphorylation. Immunoblot analysis with AMPK siRNA and SIRT1 siRNA in HUVECs and EC-specific AMPKα2 knockout mice showed that AMPK phosphorylation of cortactin primes SIRT1 deacetylation in response to shear stress. Immunoblot analyses with cortactin siRNA in HUVECs, phospho-deficient T401A and phospho-mimetic T401D mutant, or aceto-deficient (9K/R) and aceto-mimetic (9K/Q) showed that cortactin regulates eNOS activity. Confocal imaging and sucrose-density gradient analyses revealed that the phosphorylated/deacetylated cortactin translocates to the EC periphery facilitating eNOS translocation from lipid to nonlipid raft domains. Knockdown of cortactin in vitro or genetic reduction of cortactin expression in vivo in mice substantially decreased the eNOS-derived NO bioavailability. In vivo, atherosclerotic lesions increase in ApoE−/−/cortactin+/− mice, when compared with ApoE−/−/cortactin+/+ littermates.
Conclusions
AMPK phosphorylation of cortactin followed by SIRT1 deacetylation modulates the interaction of cortactin and cortical actin in response to shear stress. Functionally, this AMPK/SIRT1 coregulated cortactin-F-actin dynamics is required for eNOS subcellular translocation/activation and is atheroprotective.
Keywords: AMPK, SIRT1, Cortactin, eNOS, atherosclerosis
Graphical abstract
Introduction
The pattern of local blood flow in the arterial tree affects the endothelial phenotype. Blood flow in the straight section of the arterial tree is atheroprotective, which exerts anti-inflammatory, anti-oxidative, and anti-proliferative effects on vascular endothelial cells (ECs).1-3 AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1), activated by various metabolic and environmental stresses, are two key molecules in ECs regulated by atheroprotective flow. In vitro, pulsatile shear stress (PS), mimicking atheroprotective flow, activates endothelial nitric oxide synthase (eNOS) through AMPK and SIRT1.4 Flow regulation of AMPK and SIRT1 in relation to the atheroprotective phenotype is evidenced in mice with ablated AMPKα2 or SIRT1. These mice, when crossed with ApoE−/−, have enhanced atherosclerosis both in atheroprone and atheroprotective regions of the aorta.5 AMPK and SIRT1 synergistically regulate common downstream targets, as exemplified by the coregulation of eNOS in ECs and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) in myocytes.4,6 Here, AMPK phosphorylation of eNOS and PGC1α primes their deacetylation by SIRT1.
Cortactin is a ubiquitously expressed protein involved in cortical actin assembly/nucleation and dynamic rearrangement of actin.7-9 As a scaffold protein, the N-terminal acidic domain (NTA) of cortactin binds to the actin-related protein 2/3 (Arp2/3) complex. The repeat region binds to F-actin. The proline-rich domain is phosphorylated by various kinases, and the C-terminal Src homology 3 (SH3) domain interacts with neuronal Wiskott–Aldrich Syndrome protein (N-WASP) and WASP-interacting protein. In ECs, cortactin is functionally linked to cytoskeletal remodeling, angiogenesis, leukocyte transmigration, and vascular permeability.10-13 In terms of post-translational modifications of cortactin, the Src family kinases FER and Syk phosphorylate cortactin at Tyr-421, Tyr-466, and Tyr-482.14-17 PAK3 phosphorylates Ser-113 and ERK phosphorylates Ser-405 and Ser-418.18-20 Phosphorylation of these sites by either Tyr or Ser/Thr kinase is functionally linked to actin dynamics and assembly. Moreover, SIRT1/2 can interact with and deacetylate cortactin to affect its binding to F-actin.21
Initial localization of eNOS in the lipid rafts of the EC membrane is essential for eNOS activation. Upon exposure to shear stress, eNOS translocates from caveolae to the cytoplasm, where eNOS is phosphorylated by Akt.22-24 Reduction of eNOS in caveolae by cholesterol depletion or inhibition of eNOS myristoylation decreases stimulus-induced eNOS activation.25-27 Much evidence indicates that the interaction of caveolae with actin-based cytoskeleton and the dynamic remodeling of F-actin affect eNOS activity.28,29 Indeed, both cytochalasin B disruption and phalloidin stabilization of F-actin diminish the flow-induced NO release in the rabbit abdominal aorta.30 Additionally, several actin-binding proteins, including dynamin 2, NOSIP, and NOSTRIN are involved in eNOS trafficking and activity.31-33 Although cortactin has been reported to translocate to the cell periphery upon cortical actin assembly in response to shear stress,10 the involvement of cortactin in shear stress-modulated eNOS and EC function is unknown.
Cortactin has both AMPK phosphorylation and SIRT1 deacetylation consensus sequences.21,34,35 This report examines the hypothesis that cortactin is coregulated by AMPK and SIRT1 similar to their regulation of eNOS and PGC1α. We found that AMPK and SIRT1 not only coregulate cortactin but also play a key role in the structural and functional integrity of caveolae and are essential for shear-stress activation of eNOS. Hence, decreased expression of cortactin in the vascular wall hampers eNOS-derived NO bioavailability and increases atherosclerosis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
AMPK phosphorylates cortactin Thr-401
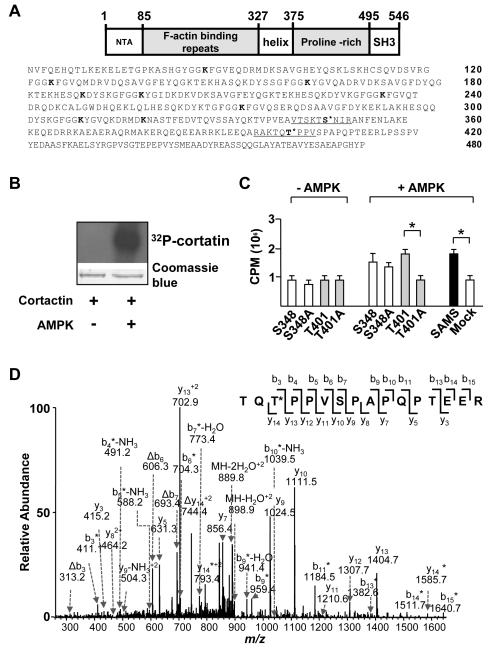
A genome-wide bioinformatics analysis for the consensus sequence of AMPK revealed that cortactin Ser-348 and Thr-401 located in the helix and proline-rich domains, respectively, could be AMPK phosphorylation sites (Figure 1A).36 Interestingly, these 2 sites are conserved among various mammalian species (Figure I in the online-only Data Supplement). Incubation of [γ-32P]ATP with recombinant cortactin and AMPK increased the level of 32P-labeled cortactin (Figure 1B), which suggested that AMPK can phosphorylate cortactin in vitro. To validate AMPK can phosphorylate cortactin Ser-348 and Thr-401, the degree of AMPK phosphorylation of 2 oligopeptides composed of the amino acids flanking Ser-348 and Thr-401 was compared with the SAMS peptide, the canonical AMPK substrate, as well as peptides with Ala substituted at the putative sites of phosphorylation (i.e., S348A and T401A). We found that AMPK phosphorylated the 2 “wild-type” cortactin peptides to the same extent as the positive-control SAMS peptide. The Ala substitution of Thr-401, but not Ser-348, decreased 32P incorporation to the level observed for samples without AMPK (Figure 1C), which suggests that Thr-401 could be an AMPK phosphorylation site. To determine further the site of AMPK phosphorylation of cortactin, we treated human umbilical vein endothelial cells (HUVECs) with resveratrol (RSV), a polyphenol activating/inducing AMPK and SIRT1, or 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK activator. Cortactin was immunoprecipitated from the protein extracts and subjected to tandem mass spectrometry analysis. By comparing the m/z values for the observed fragment ions with those calculated for the phosphorylated Thr-401-bearing tryptic peptide (Table I in the online-only Data Supplement), mass spectrometry results indicated that Thr-401 in cortactin was phosphorylated by AMPK in both RSV and AICAR-treated ECs (Figure 1D and Figure II in the online-only Data Supplement).
Figure 1. AMPK phosphorylates cortactin at Thr-401.
(A) Schematic of the structural domains and amino acid sequence of human cortactin. Underlined segments indicate sequences of oligopeptide prepared for in vitro kinase assays. The putative AMPK phosphorylation sites of cortactin are located at Ser-348 and Thr-401. Bolded lysine (K) residues (Lys-87, -124, -161, -189, -198, -235, -272, -309, and -319) indicate predicted SIRT1 deacetylation sites within the F-actin binding repeats. (B) SDS-PAGE of recombinant cortactin and [γ-32P]ATP in the presence or absence of AMPKα2. Upper panel: autoradiograph of gel. Lower panel: photograph of Coomassie blue-stained gel. (C) Bar graph (mean ± SEM from 3 independent experiments) of 32P incorporation into peptides containing flanking sequences adjacent to cortactin Ser-348 and Thr-401 and SAMS peptide in the presence and absence of AMPKα2. Mock control indicates the absence of added peptide. ‘*’ indicates P < 0.05 between indicated groups. (D) Product-ion spectrum (MS/MS) of the phosphorylated tryptic peptide corresponding to residues 399-414 (TQTPPVSPAPQPTEER) from immunoprecipitated cortactin in HUVECs treated with RSV. The asterisk indicates that an ion bears a phosphate group, and neutral loss of an H3PO4 is represented by ‘Δ’.
AMPK/SIRT1 coregulate cortactin
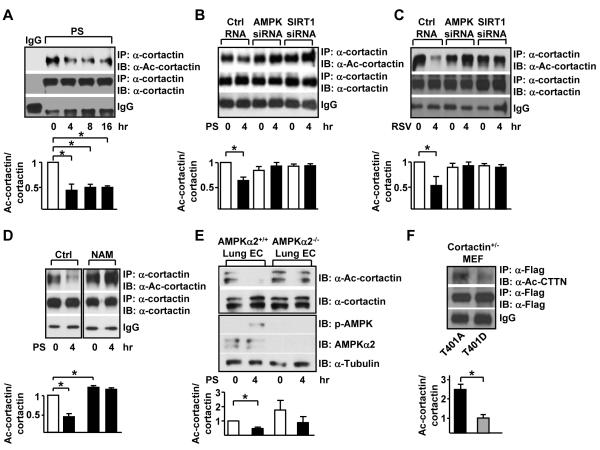
Given that PS activates both AMPK and SIRT1 in ECs and SIRT1 deacetylates cortactin,4,21 we examined whether cortactin can be coregulated by AMPK and SIRT1 in ECs responding to PS. HUVECs were subjected to PS for various times followed by immunoprecipitation of cortactin and immunoblotting for acetylated cortactin. Deacetylation of cortactin was evident as early as 4 hr and the PS-reduced cortactin acetylation persisted for at least 16 hr (Figure 2A). Knockdown of either AMPK or SIRT1 by siRNA blocked PS-reduced cortactin acetylation (Figure 2B). Similar to PS, RSV treatment decreased cortactin acetylation in HUVECs (Figure 2C). Knockdown of AMPK or SIRT1 also blocked the resveratrol-reduced cortactin acetylation (Figure 2C). To further investigate the AMPK/SIRT1 coregulation of cortactin, HUVECs were treated with nicotinamide (NAM), a SIRT1 specific inhibitor prior to PS stimulation. Deacetylation of cortactin occurred at 4 hr under PS. However, cortactin remained acetylated if HUVECs were pre-treated with NAM (Figure 2D). Moreover, the PS-reduced cortactin acetylation was not observed in lung ECs isolated from EC-specific AMPKα2 −/− mice (Figure 2E). Next, we mutated cortactin Thr-401 to Ala (T401A) or Asp (T401D), the respective phospho-deficient and -mimetic mutants. When expressed in cortactin heterozygous (cortactin+/−) MEFs, T401D mutant was less acetylated when compared with T401A mutant (Figure 2F). Together, the results in Figure 2 suggest that AMPK phosphorylation of cortactin Thr-401 primes cortactin deacetylation by SIRT1.
Figure 2. AMPK phosphorylation of cortactin primes SIRT1 deacetylation.
(A) HUVECs underwent pulsatile flow (PS, 12 ± 4 dyn/cm2) for indicated times. (B) HUVECs were transfected with control RNA, AMPK siRNA, or SIRT1 siRNA. The transfected cells were kept under static conditions or subjected to PS for 4 hr. (C) AMPK and SIRT1 were activated with resveratrol (RSV) for 4 hr. (D) HUVECs were treated with nicotinamide (NAM, 5 mM) to inhibit SIRT1 activity. (E) Lung endothelial cells isolated from EC-specific AMPKα2 knockout mice (AMPKα2 −/−) and their WT littermates (AMPKα2 +/+) were kept under static conditions or subjected to PS for 4 hr. (F) Cortactin+/− MEFs were transfected with Flag-tagged cortactin phospho-deficient (T401A), or phospho-mimic (T401D) expression plasmid. Cortactin acetylation was determined by immunoprecipitation with α-Flag and immunoblotting with α-Ace-cortactin or α-Flag. Upper subpanels are immunoblots with anti-α-cortactin mAbs and anti-α-Ac-cortactin polyclonal antibody. Data are mean ± SEM of 3 independent experiments. ‘*’ indicates P < 0.05.
Cortactin regulates eNOS activity
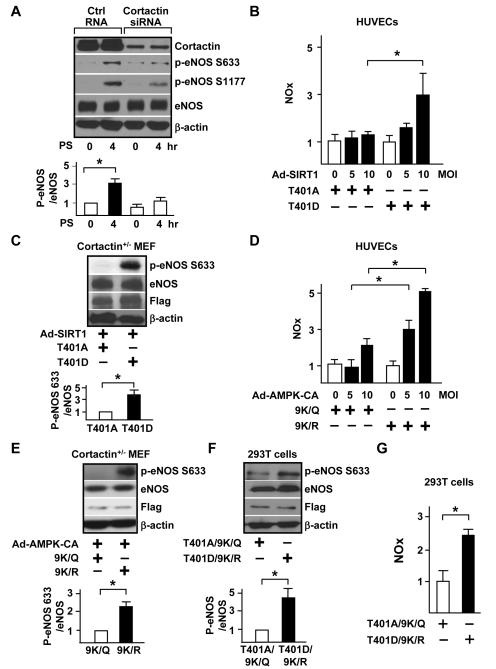
Because AMPK and SIRT1 coregulate cortactin at the post-translational level and because shear stress is a physiological stimulation regulating eNOS, we next investigated the involvement of cortactin in PS-activated eNOS. PS increased eNOS phosphorylation at Ser-633 and Ser-1177 (Figure 3A), which is indicative of increased eNOS activity. This increased phosphorylation was attenuated by cortactin knockdown, which suggests that PS induction of eNOS is cortactin-dependent. Next, we examined whether cortactin-dependent eNOS activation is regulated through AMPK and SIRT1. HUVECs were transfected with cortactin phospho-deficient T401A or phospho-mimic T401D mutants, then infected with adenovirus overexpressing SIRT1 (Ad-SIRT1) to mimic PS induction of SIRT1. Overexpression of SIRT1 increased NO production in cells expressing cortactin T401D, but not in cells transfected with cortactin T401A (Figure 3B). To further demonstrate that eNOS activity was modulated by cortactin in an AMPK/SIRT1-dependent manner, we overexpressed eNOS with the cortactin T401A or T401D mutant in cortactin+/− MEFs and infected adenovirus overexpressing SIRT1 to simulate PS activation of AMPK/SIRT1. Cells transfected with cortactin T401D and infected with Ad-SIRT1 showed higher eNOS activity, as indicated by an increase of phosphorylation at eNOS Ser-633 (Figure 3C). Likewise, overexpression of constitutively active AMPK (Ad-AMPK-CA) increased NO production in HUVECs transfected with aceto-deficient mutants (cortactin 9K/R), but not aceto-mimetic mutants (cortactin 9K/Q) (Figure 3D). Furthermore, cortactin+/− MEFs overexpressing cortactin 9K/R infected with Ad-AMPK-CA showed increased eNOS phosphorylation. In contrast, MEFs overexpressing cortactin 9K/Q mutants did not respond to Ad-AMPK-CA transfection (Figure 3E). To further delineate the role of AMPK/SIRT1 in activating eNOS via cortactin, we co-transfected cortactin T401D/9K/R (phospho-mimetic/aceto-deficient mutant) or cortactin T401A/9K/Q (phospho-deficient/aceto-mimetic mutant) with a plasmid encoding eNOS in human embryonic kidney 293 (HEK293) cells to detect NO production. Cells overexpressing cortactin T401D/9K/R showed increased eNOS phosphorylation as well as NO production (Figure 3F and 3G), which further confirms that phosphorylated/deacetylated cortactin modulates eNOS activity.
Figure 3. AMPK/SIRT1-CTTN regulates eNOS activity.
(A) HUVECs were transfected with control RNA or cortactin siRNA. Transfected cells were kept under static conditions or subjected to PS for 4 hr. Immunoblotting was performed with antibodies as indicated. Data are ratios of p-eNOS 633 to eNOS. (B) HUVECs were transfected with cortactin T401A or T401D plasmid and infected with adenovirus overexpressing SIRT1 (Ad-SIRT1) at 0, 5, and 10 multiplicity of infection (MOI). Data are relative mean ± SEM of NO production, with cells transfected with T401A set to 1. (C) Cortactin+/− MEF cells were transfected with cortactin T401A or T401D plasmid and infected with Ad-SIRT1. (D) HUVECs were transfected with cortactin 9K/Q or 9K/R plasmid and infected with adenovirus overexpressing a constitutive activated form of AMPKα (Ad-AMPK-CA) at 0, 5, and 10 MOI. (E) Cortactin+/− MEFs were transfected with cortactin 9K/Q or 9K/R plasmid and infected with Ad-AMPK-CA. (F, G) HEK293T cells were transfected with cortactin phosphorylation/deacetylation mimic T401D9K/R or dephosphorylation/acetylation mimic T401A9K/Q. Upper panels in A, C, E, and F are immunoblots with indicated antibodies and data are mean ± SEM from 3 independent experiments. eNOS phosphorylation and NO production were assessed in (F) and (G), respectively. ‘*’ indicates P < 0.05.
AMPK/SIRT1-cortatin regulation of eNOS trafficking
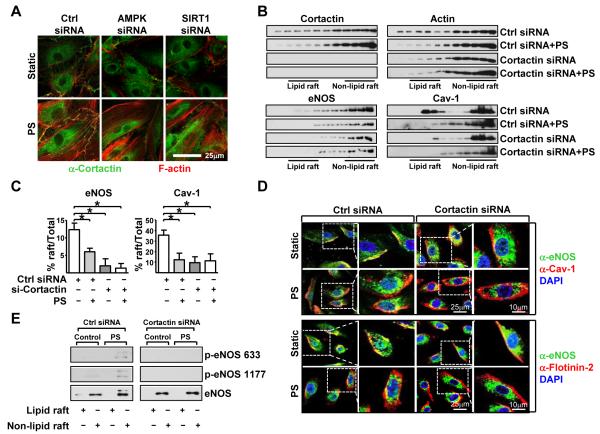
Because the integrity of actin cytoskeletal is required for shear stress-activated eNOS and deacetylated cortactin increases its interaction with F-actin,34 we next examined the impact of AMPK/SIRT1 on the cortactin-actin association in relation to eNOS translocation and activity. We first knocked down AMPK or SIRT1 with siRNA and then examined the cortical-actin association under PS. The association of cortactin and F-actin was indicated by the degree of colocalization observed by using double immunofluorescent staining. Confocal microscopy showed that PS enhanced the cortactin interaction with cortical actin, and siRNA knockdown of AMPK or SIRT1 inhibited this interaction (Figure 4A).
Figure 4. AMPK/SIRT1-CTTN regulates eNOS trafficking.
(A) Confocal images of HUVECs transfected with control RNA, AMPK siRNA, or SIRT1 siRNA and then held under static conditions or PS for 4 hr. Cells were fixed and immunostained with anti-cortactin (green) and rhodamine-phalloidin (red), which binds to F-actin. PS-induced colocalization of cortactin and F-actin at the cortical regions is seen as the additive yellow color. (B, C, D) HUVECs were transfected with control RNA or cortactin siRNA and then kept under static conditions or subjected to PS. In (B), cell lysates were fractionized by sucrose-density gradient ultracentrifugation. The distribution of cortactin, actin, eNOS, and Cav-1 in the lipid raft fractions (3-6) and nonlipid raft fractions (9-12) was revealed by immunoblotting with antibodies against cortactin, actin, eNOS, and Cav-1. Statistical analysis of eNOS and Cav-1 residence in lipid raft was shown in (C). (D) Confocal images of HUVECs under static conditions or subjected to PS and transfected with control RNA or cortactin siRNA. Cells were immunostained with anti-eNOS (green) and anti-flot-2 (red) antibodies. (E) Immunoblot of the pooled lipid raft and nonlipid raft fractions analyzed with anti-phospho-eNOS and anti-eNOS antibodies.
Cortical actin is important for the formation and function of caveolae.37,38 eNOS activation by shear stress requires eNOS translocation from caveolae to the cytoplasm, and disruption of F-actin or lipid rafts blunts shear stress activation of eNOS.27,39 Because AMPK/SIRT1 coregulated cortactin is functionally related to eNOS activity (demonstrated in Figure 3), we next investigated the role of cortactin-actin assembly in eNOS trafficking in response to PS. Sucrose-density gradient analysis showed that under static conditions, eNOS, cortactin, actin, and caveolin-1 (Cav-1, a caveolae marker) are distributed in both lipid raft and nonlipid raft fractions (Figure 4B). PS shifted eNOS, cortactin, actin, and Cav-1 to from lipid raft to nonlipid raft fractions. Of note, cortactin siRNA knockdown stimulated eNOS, actin, and Cav-1 to localize to the nonlipid raft fractions regardless of static or PS conditions (Figure 4B and C). Translocation of eNOS, actin, and Cav-1 out of the lipid rafts was also observed in ECs treated with cytochalasin D (Figure III in the online-only Data Supplement), which disrupts F-actin-based cytoskeleton, and in cells with AMPK or SIRT1 knocked down (Figure IV in the online-only Data Supplement). In addition, cortactin-dependent eNOS compartmentalization was examined by immunofluorescence microscopy. Under static conditions, eNOS and flot-2 (a lipid raft marker), as well as eNOS and Cav-1, colocalized (Figure 4D), which indicates that a significant portion of eNOS in ECs was in lipid rafts. Under PS, eNOS was largely dissociated from lipid rafts. Cortactin knockdown shifted eNOS out of lipid rafts both under static conditions and PS. To further assess whether cortactin-dependent eNOS compartmentalization contributes to eNOS activity, lipid raft, as well as nonlipid raft fractions, were pooled and blotted for eNOS and phospho-eNOS. eNOS was found in the nonlipid raft fractions in HUVECs under PS, accompanied by increased eNOS phosphorylation at Ser-633 (Figure 4E). Importantly, cortactin knockdown abrogated eNOS translocation and activation in response to PS stimuli (Figure 4E).
Cortactin-dependent eNOS activation in the vascular wall
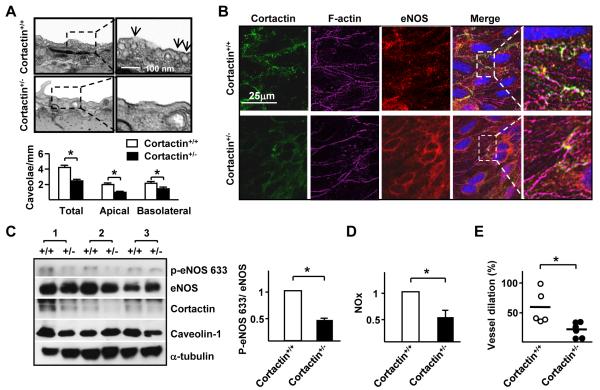
Because the systemic knockout of cortactin is embryonic lethal,40 we used cortactin+/− mice and their cortactin+/+ littermates to study the role of AMPK/SIRT1 coregulation of cortactin in the vascular wall. One possible mechanism for cortactin regulation of eNOS trafficking is that cortactin is required for caveolae formation. To test this hypothesis, we compared endothelial caveolae in cortactin+/+ with that in cortactin+/− mice. Electron micrographs shown in Figure 5A reveal that endothelium from the wild-type animals had approximately 2 ± 0.2/μm cavelolae in both apical and basolateral membranes. However, the amount of cavelolae was greatly reduced in the cortactin+/− endothelium (1 ± 0.1/μm in apical and 1.5 ± 0.1/μm in basolateral membranes). Cortactin, F-actin, and eNOS subcellular location in the vascular wall was compared by en face staining of endothelium in aortas from cortactin+/+ and cortactin+/− mice. Thoracic aorta of cortactin+/+ mice showed cortactin, F-actin, and eNOS colocalization at cell-cell junctions of endothelium but to a much lesser extent in corresponding regions of cortactin+/− mice (Figure 5B). Immunoblotting with phospho-eNOS and cortactin antibodies showed decreased eNOS phosphorylation in cortactin+/− aortas (Figure 5C). The decreased eNOS phosphorylation in cortactin+/− mice was associated with both impaired NO production (Figure 5D) and flow-induced vessel dilation (Figure 5E).
Figure 5. Cortactin-dependent eNOS localization in the mouse aorta.
(A) EM images of aorta isolated from cortactin+/− mice and cortactin+/+ littermates. Bar graph in lower panel shows the levels of caveolae (mean ± SEM from 3 independent experiments) in listed areas. (B) En face immunostaining of cortactin, eNOS, and F-actin in thoracic aortas isolated from cortactin+/+ and cortactin+/− mice. The additive yellow color indicates colocalization of cortactin, eNOS, and F-actin. (C, D) Aortas were isolated from cortactin+/+ and cortactin+/− mice (n=3 in each group) and tissue was lyzed. In (C), aortic extracts were analyzed by immunoblotting with indicated antibodies. In (D) NO production was measured. Data are mean ± SEM from at least 3 sets of experiments. (E) Flow-induced vasodilation ex vivo in cerebellar microvessels from cortactin+/+ (n = 5) and cortactin+/− (n = 6) mice pre-treated with phenylephrine (1 μM). The vessel dilation % is defined as the percentage of the diameter change of the flow-induced dilation compared with the diameter change of the phenylephrine-induced constriction. ‘*’ indicates P < 0.05.
Cortactin exerts an atheroprotective effect
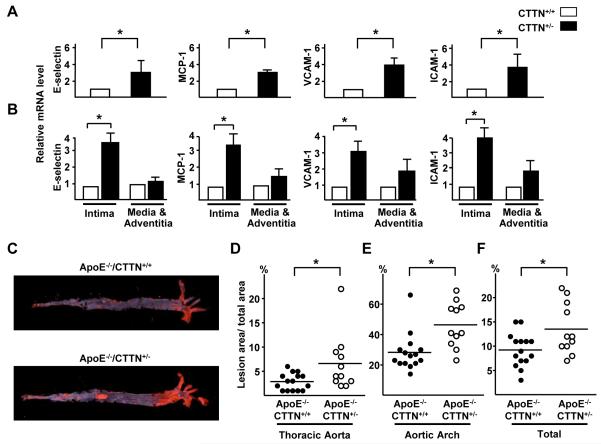
To determine whether the lower level of NO in vessels of cortactin+/− mice negatively affected endothelial function, we compared the expression of chemoattractant and adhesion molecules in the thoracic aortas of cortactin+/+ and cortactin+/− mice. The mRNA levels of E-selectin, monocyte chemoattractant protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) were higher in cortactin+/− than cortactin+/+ mouse aortas (Figure 6A). This difference was mainly found in the intima (endothelium) but not media and adventitia (Figure 6B). To determine whether the endothelial dysfunction in cortactin+/− mice were atherogenic, we introduced an ApoE−/− background into cortactin+/− mice to generate ApoE−/−/cortactin+/− mice and their ApoE−/−/cortactin+/+ littermates. The two groups were fed an atherogenic diet for 10 weeks and atherosclerotic lesions in aortas was quantified and compared. The mean lesion area in thoracic aortas was ~1.5-fold larger for ApoE−/−/cortactin+/− than ApoE−/−/cortactin+/+ mice (6.5 ± 1.7% vs. 3.0 ± 0.4%) (Figure 6C and 6D). Additionally, the aortic arch lesion area and total lesion area (the sum of aortic arch, thoracic aorta, and abdominal aorta) were larger in the ApoE−/−/cortactin+/− than in the ApoE−/−/cortactin+/+ mice (46.4 ± 4.5% vs. 24.4 ± 3.2% and 13.6 ± 5.3% vs. 9.3 ± 3.5%) (Figure 6C, 6E, and 6F). Because the serum lipid profiles and M1 and M2 polarization in the isolated macrophages from the two groups of animals were comparable (Table II and Figure V in the online-only Data Supplement), increased lesion sizes in the ApoE−/−/cortactin+/− mice should not have resulted from variations in lipoprotein metabolic or monocyte/macrophage phenotypic changes. Rather, haploid expression of cortactin in endothelium accounted for the increased atherosclerosis in ApoE−/−/cortactin+/− mice.
Figure 6. AMPK/SIRT1 co-regulation of cortactin is atheroprotective.
(A) Total mRNA levels of E-selectin, MCP-1, VCAM-1, and ICAM-1 in thoracic aortas from cortactin+/+ and cortactin+/− mice, data are mean ± SEM from 5 mice in each group. (B) Intima was further dissected from the aortic specimens and the remaining tissue was defined as media and adventitia, and the mRNA levels of E-selectin, MCP-1, VCAM-1, and ICAM-1 were then quantified. Data are mean ± SEM from 6 mice in each group. (C) ApoE−/−/cotactin+/+ mice (n = 15) and ApoE−/−/cotactin+/− (n = 11) littermates were fed on atherogenic diet for 10 weeks. Atherosclerosis in mouse aortas was stained with Oil-red O. The macrophotographs shown are representative of results from the two groups. (D, E, F) Scatterplots and quantification of percentage lesion areas (with perpendicular lines indicating mean) in the thoracic aorta, aortic arch, and a sum of the whole aorta. ‘*’ indicates P < 0.05.
Discussion
Atherosclerotic lesions develop preferentially at branches, bifurcations, and the aortic root where the endothelium is exposed to disturbed blood flow patterns. Straight segments of the arterial tree are largely athero-resistant, in part, via laminar flow-activated AMPK and SIRT1.4,5 We and others showed that AMPK and SIRT1 activate eNOS via post-translational mechanisms in ECs in response to shear stress. Shear stress activation of eNOS may rely on the integrity of actin-based cytoskeleton,30,41,42 which suggests an F-actin-related mechanism as a prerequisite for AMPK/SIRT1 phosphorylation/deacetylation of eNOS. In this report, we show that AMPK phosphorylation of cortactin followed by SIRT1 deacetylation modulates the interaction of cortactin and cortical actin. In terms of function, this AMPK/SIRT1 coregulated cortactin-F-actin dynamics directs eNOS subcellular translocation and activation.
The AMPK phosphorylation site Thr-401 is located in the proline-rich domain (residues 327-494) of cortactin and harbors several Tyr and Ser/Thr phosphorylation sites. The phosphorylation of Tyr-421, Tyr-466, and Tyr-482 by Src kinase regulates actin assembly by modulating cortactin binding to N-WASP, MLCK, and dynamin 2.43-45 ERK phosphorylation of Ser-405 and Ser-418 initiates Arp2/3-dependent actin nucleation via N-WASP.20 Additionally, recent phosphoproteomic analysis indicates that Akt phosphorylates Thr-401.46 This phosphorylation event is involved in migration and invasion of the breast cancer cell line MCF-10A. Ser/Thr versus Tyr phosphorylation of cortactin may differentially affect actin assembly, because Ser/Thr phosphorylation affects actin polymerization, whereas Tyr phosphorylation regulates focal adhesion turnover.47 Additionally, because Tyr phosphorylation alone is insufficient for subcellular translocation of cortactin responding to shear stress or a receptor agonist,10,48 the interplay of AMPK/SIRT1 on cortactin-actin assembly would be functionally different from Src tyrosine regulation. Indeed, AMPK or SIRT1 knockdown has little effect on shear stress-induced stress fiber formation and alignment (Figure 4A). This result further suggests that although AMPK/SIRT1 coregulation of cortactin-F-actin dynamics is required for eNOS activation, this mode of regulation is not involved in the actin cytoskeletal remodeling that would otherwise be regulated by ERK.47 Here, two mechanisms are likely involved in AMPK/SIRT1-induced cortactin-actin assembly. First, AMPK phosphorylation of cortactin proline-rich domain induces a conformational change of F-actin binding repeats and thus favoring SIRT1 deacetylation to promote cortical actin remodeling. AMPK phosphorylation of cortactin may also liberate SH3 domains from intra-molecular binding to the proline-rich domain. Second, SIRT1 deacetylation enables cortactin-cortical actin assembly through the positively charged Lys patch (Lys-87, -124, -161, -189, -198, -235, -272, -309, and -319). AMPK and SIRT1 also synergistically regulate eNOS and PGC1α similar to that of cortactin4,6 However, knockdown of cortactin had little effect on PGC1α expression in HUVECs (Figure VI in the online-only Data Supplement), which suggests that cortactin is not an upstream regulator of PGC1α. Thus, AMPK may phosphorylate cortactin, eNOS, and PGC1α, which primes for the deacetylation of these three molecules independently.
The assembly of cortical actin is required for the organization and localization of caveolae near the plasma membrane.49 Furthermore, F-actin association with membrane caveolae stabilizes caveolae structure and controls caveolae endocytosis.29,50-52 Here, our results suggest that caveolae formation requires cortactin, because cortactin knockdown shifts Cav-1 and actin to the nonlipid fractions (Figure 4B). In vivo, the reduced level of caveolae in the vascular wall of cortactin+/− mice (Figure 5A) suggests that cortactin is an integral part of the F-actin apparatus required to maintain caveolae in the vasculature. Knockdown of AMPK or SIRT1 abolishes cortactin-F-actin interaction and also shifts Cav-1 (a caveolae marker) to the nonlipid raft regions (Figure 4 and Figure IV in the online-only Data Supplement). Because cytochalasin D treatment displaces cortactin-actin, as well as Cav-1, from the nonlipid raft regions (Figure III in the online-only Data Supplement), caveolae formation in the lipid-rich domains may depend on AMPK/SIRT1 regulation of the cortactin-F-actin complex. A possible mechanism is that AMPK phosphorylation followed by SIRT1 deacetylation enhances the interaction between the Lys-rich patch of cortactin with F-actin. Such an enhanced cortactin-F-actin interaction could lead to the translocation of the cortical actin complex with Cav-1 and eNOS in the lipid raft.
The AMPK/SIRT1-dependent regulation of caveolae via cortactin-F-actin is required for eNOS activation. The process can be activated mechanically or pharmacologically (i.e., by PS or resveratrol), which suggests that cortactin-F-actin assembly is a common component for stimuli that increase eNOS-derived NO bioavailability. Much evidence demonstrates that eNOS trafficking from membrane caveolae to the cytoplasm is required for its activation,25,27,38,53 but our data reveal that this subcellular localization and translocation of eNOS depends on AMPK/SIRT1 coregulation of cortactin. The most likely explanation for this is that eNOS translocation requires cortactin-F-actin in an AMPK/SIRT1-dependent manner. This postulation is supported by observations that cortactin T401D/9K/R (a gain-of-function mutant) is associated with higher levels of NO (Figure 3F and 3G) and that F-actin disruption or caveolae diminishment impairs eNOS activity.26,27,30 At the tissue level, metformin administration increased the eNOS translocation to the nonlipid raft in mouse ECs (Figure VIIA in the online-only Data Supplement). Furthermore, Cav-1 dissociated with eNOS in response to metformin treatment demonstrated by en face confocal immunostaining (Figure VIIB in the online-only Data Supplement). In relation to atherosclerosis, AMPK/SIRT1 coregulation of cortactin-F-actin affects endothelial function preceding atherosclerosis, which would be due, in part, to impaired eNOS activity. The consistent increase in atherosclerosis in the thoracic aorta of ApoE−/−/cortactin+/−(Figure 6), ApoE−/−/AMPKα2−/−, and ApoE−/−/EC-SIRT1−/− mice suggests that the flow-regulated cortactin-F-actin is associated with AMPK and SIRT1 activity.5 Besides cortactin, cortical-actin complexes contain Arp2/3, N-WASP/WAVE, and dynamin-2. Whether these proteins affect ECs in the same manner as cortactin warrants further study.
Although vascular smooth muscle cells (VSMCs) in the vasculature are not in direct contact with blood flow, the AMPK/SIRT1-cortactin signaling seems to be functional in VSMCs in two ways. First, AMPK/SIRT1-cortactin may positively regulate the redox state of VSMCs because AMPKα2 or cortactin knockdown results in an increased ROS level and NOX4 expression with concurrent decrease in SOD1 expression (Figure VIII in the online-only Data Supplement). If so, the beneficial effect of AMPK and SIRT1 in VSMCs would be in part mediated through cortactin.54,55 Second, AMPK/SIRT1-cortactin signaling in ECs seems to affect the homeostasis of VSMCs. Supporting this postulation, ECs treated with metformin or EC transfected with T401D are associated with an increased cGMP level in the conditioned media (Figure IXB in the online-only Data Supplement), as well as a decreased ROS level in the co-cultured VSMCs (Figure IXC in the online-only Data Supplement).
What appears to argue against the above conclusions is that endothelial-specific overexpression of Cav-1 with an attendant increase in caveolae is atherogenic, and Cav-1 knockout in the ApoE-null background decreases atherosclerosis.56-58 However, endothelium from caveolin−/− mice have higher levels of NO, which is usually predictive of atheroprotection.58,59 Thus, caveolae abundance resulting from gene manipulations is not predictive of atherogenesis. Caveolae are signaling hubs harboring an array of both pro- and anti-inflammatory molecules. The dynamic interactions of these caveolae-associated molecules with the cortical-actin complex that includes cortactin may be more important for modulation of the endothelial phenotype than level of Cav-1. As discussed above, cortactin+/− mice with decreased levels of caveolae (Figure 5A) exhibit a pro-inflammatory and dysfunctional endothelial phenotype. In contrast, eNOS activity is reduced and the flow-induced dilation is impaired in vessels from cortactin+/− mice (Figure 5C-5E). Furthermore, ApoE−/−/cortactin+/− mice show increased atherosclerosis (Figure 6C). Because atheroprotective flow-activated AMPK and SIRT1 can directly modulate eNOS activity via post-translational modifications,4,5 our findings provide added complexity to AMPK/SIRT1 regulation of eNOS that involves cortactin-F-actin dynamics.
Previous studies demonstrate that the cortactin-dependent pathways are activated by sphingosine 1-phosphate (S1P) and that S1P is associated with high density lipoprotein (HDL).48,60-62 Related to these findings, it was indicated that synthetic S1P analogs ameliorate atherosclerosis.63,64 The clinical implication of the current study, together with those previous, is that the athero-protective effect exerted by AMPK/SIRT1 and S1P signaling maybe mediated through cortactin.
Supplementary Material
Highlights.
AMPK phosphorylates cortactin at T401.
AMPK/SIRT1 coregulation of cortactin contributes to eNOS activation.
Deficiency of cortactin results in the increase of atherosclerosis in AopE−/− mice.
Acknowledgments
Sources of Funding
This work was supported in part by NIH research grants R01HL105318, R01HL106579, and R01HL108735 (JS), R01ES019873 (YW).
Abbreviations
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- AMPK
AMP-activated protein kinase
- Arp2/3
actin-related protein 2/3
- Cav-1
caveolin-1
- ECs
endothelial cells
- eNOS
endothelial nitric oxide synthase
- Flot-2
flotillin 2
- ICAM-1
intercellular adhesion molecule 1
- MCP-1
monocyte chemoattractant protein 1
- MEFs
murine embryonic fibroblasts
- N-WASP
neuronal Wiskott–Aldrich Syndrome protein
- NTA
N-terminal acidic domain
- HUVECs
human umbilical vein endothelial cells
- PGC1α
peroxisome proliferator-activated receptor γ coactivator 1 α
- PS
pulsatile shear stress
- SH3
Src homology 3
- SIRT1
sirtuin 1
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Disclosures
None.
References
- 1.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 2.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen L, Chen Z, Zhang F, Cui X, Sun W, Geary GG, Wang Y, Johnson DA, Zhu Y, Chien S, Shyy JY. Ca2+/calmodulin-dependent protein kinase kinase beta phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E2420–E2427. doi: 10.1073/pnas.1309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem. J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil. Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am. J. Respir. Cell Mol. Biol. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 11.Kaluza D, Kroll J, Gesierich S, Yao TP, Boon RA, Hergenreider E, Tjwa M, Rössig L, Seto E, Augustin HG, Zeiher AM, Dimmeler S, Urbich C. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011;30:4142–4156. doi: 10.1038/emboj.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ. Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 13.Schnoor M, Lai FP, Zarbock A, Kläver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J. Exp. Med. 2011;208:1721–1735. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 16.Kim L, Wong TW. Growth Factor-dependent Phosphorylation of the Actin-binding Protein Cortactin Is Mediated by the Cytoplasmic Tyrosine Kinase FER. J. Biol. Chem. 1998;273:23542–23548. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- 17.Gallet C, Rosa JP, Habib A, Lebret M, Lévy-Tolédano S, Maclouf J. Tyrosine Phosphorylation of Cortactin Associated with Syk Accompanies Thromboxane Analogue-induced Platelet Shape Change. J. Biol. Chem. 1999;274:23610–23616. doi: 10.1074/jbc.274.33.23610. [DOI] [PubMed] [Google Scholar]
- 18.Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS. Phosphorylation of cortactin by p21-activated kinase. Arch. Biochem. Biophys. 2006;456:183–193. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DH, Sutherland RL, Daly RJ. Signaling Pathways and Structural Domains Required for Phosphorylation of EMS1/Cortactin. Cancer Res. 1999;59:5376–5385. [PubMed] [Google Scholar]
- 20.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src Phosphorylation of Cortactin Acts as a Switch On-Switch Off Mechanism That Controls Its Ability To Activate N-WASP. Mol. Cell. Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J. Biol. Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 23.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 24.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of Endothelial Nitric-oxide Synthase to the Cytoplasmic Face of the Golgi Complex or Plasma Membrane Regulates Akt- Versus Calcium-dependent Mechanisms for Nitric Oxide Release. J. Biol. Chem. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 25.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J. Biol. Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez E, Kou R, Lin AJ, Golan DE, Michel T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J. Biol. Chem. 2002;277:39554–39560. doi: 10.1074/jbc.M207299200. [DOI] [PubMed] [Google Scholar]
- 27.Lungu AO, Jin ZG, Yamawaki H, Tanimoto T, Wong C, Berk BC. Cyclosporin A inhibits flow-mediated activation of endothelial nitric-oxide synthase by altering cholesterol content in caveolae. J. Biol. Chem. 2004;279:48794–48800. doi: 10.1074/jbc.M313897200. [DOI] [PubMed] [Google Scholar]
- 28.Gowrishankar K, Ghosh S, Saha S, C R, Mayor S, Rao M. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell. 2012;149:1353–1367. doi: 10.1016/j.cell.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutcheson IR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br. J. Pharmacol. 1996;118:720–726. doi: 10.1111/j.1476-5381.1996.tb15459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J. Biol. Chem. 2001;276:14249–14256. doi: 10.1074/jbc.M006258200. [DOI] [PubMed] [Google Scholar]
- 32.Dedio J, König P, Wohlfart P, Schroeder C, Kummer W, Müller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 2002;99:17167–17172. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 36.Marin TL, Gongol B, Martin M, King S, Smith L, Johnson DA, Subramaniam S, Chien S, Shyy JY. Identification of AMP-activated protein kinase targets by a consensus sequence search of the proteome. BMC Syst Biol. 2015;9:13. doi: 10.1186/s12918-015-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell. 2000;11:325–337. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J. Biol. Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J. Clin. Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D, Zhang H, Blanpied TA, Smith E, Zhan X. Cortactin is implicated in murine zygotic development. Exp. Cell Res. 2010;316:848–858. doi: 10.1016/j.yexcr.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Searles CD, Ide L, Davis ME, Cai H, Weber M. Actin cytoskeleton organization and posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ. Res. 2004;95:488–495. doi: 10.1161/01.RES.0000138953.21377.80. [DOI] [PubMed] [Google Scholar]
- 42.Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, van Cappellen GW, Bos J, Slager CJ, Duncker DJ, van der Steen AF, de Crom R, Krams R. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 43.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 2002;298:511–519. doi: 10.1016/s0006-291x(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 45.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, Renuse S, Sahasrabuddhe NA, et al. Activation of diverse signalling pathways by oncogenic PIK3CA mutations. Nat Commun. 2014;5:4961. doi: 10.1038/ncomms5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruchten AE, Krueger EW, Wang Y, McNiven MA. Distinct phospho-forms of cortactin differentially regulate actin polymerization and focal adhesions. Am. J. Physiol., Cell Physiol. 2008;295:C1113–C1122. doi: 10.1152/ajpcell.00238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J. Biol. Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 49.Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell. Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 50.Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat. Rev. Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 51.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 52.Muriel O, Echarri A, Hellriegel C, Pavón DM, Beccari L, Del Pozo MA. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J. Cell. Sci. 2011;124:2763–2776. doi: 10.1242/jcs.080804. [DOI] [PubMed] [Google Scholar]
- 53.Feron O, Saldana F, Michel JB, Michel T. The Endothelial Nitric-oxide Synthase-Caveolin Regulatory Cycle. J. Biol. Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- 54.Thompson AM, Martin KA, Rzucidlo EM. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS One. 2014;9:e85495. doi: 10.1371/journal.pone.0085495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Liang B, Viollet B, Zou MH. Inhibition of the AMP-Activated Protein Kinase-α2 Accentuates Agonist-Induced Vascular Smooth Muscle Contraction and High Blood Pressure in Mice. Hypertension. 2011;57:1010–1017. doi: 10.1161/HYPERTENSIONAHA.110.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Hernando C, Yu J, Davalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 58.Fernández-Hernando C, Yu J, Suárez Y, Rahner C, Dávalos A, Lasunción MA, Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J. Clin. Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem Cell Biol. 2006;126:297–304. doi: 10.1007/s00418-006-0143-z. [DOI] [PubMed] [Google Scholar]
- 61.Arce FT, Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MF, Lal R, Garcia JG, Dudek SM. Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin. Biophys J. 2008;95:886–894. doi: 10.1529/biophysj.107.127167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- 63.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen 62 EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;11:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 64.Poti F, Gualtieri F, Sacchi S, Weissen-Plenz G, Varga G, Brodde M, Weber C, Simoni M, Nofer JR. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R−/− mice. Arterioscler Thromb Vasc Biol. 2013;33:1505–1512. doi: 10.1161/ATVBAHA.113.301347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.