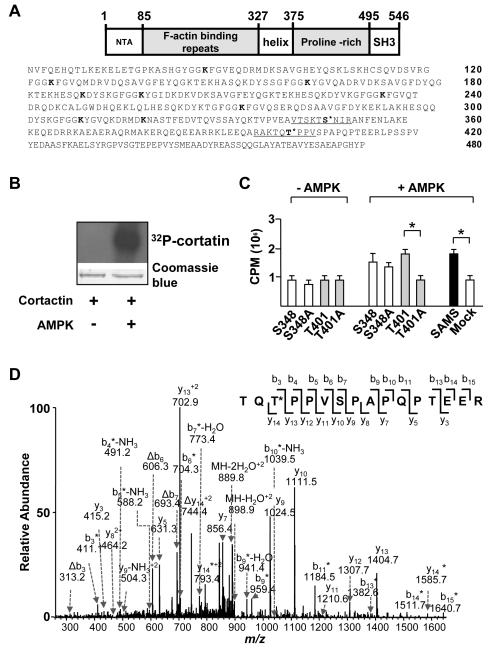
Figure 1. AMPK phosphorylates cortactin at Thr-401.
(A) Schematic of the structural domains and amino acid sequence of human cortactin. Underlined segments indicate sequences of oligopeptide prepared for in vitro kinase assays. The putative AMPK phosphorylation sites of cortactin are located at Ser-348 and Thr-401. Bolded lysine (K) residues (Lys-87, -124, -161, -189, -198, -235, -272, -309, and -319) indicate predicted SIRT1 deacetylation sites within the F-actin binding repeats. (B) SDS-PAGE of recombinant cortactin and [γ-32P]ATP in the presence or absence of AMPKα2. Upper panel: autoradiograph of gel. Lower panel: photograph of Coomassie blue-stained gel. (C) Bar graph (mean ± SEM from 3 independent experiments) of 32P incorporation into peptides containing flanking sequences adjacent to cortactin Ser-348 and Thr-401 and SAMS peptide in the presence and absence of AMPKα2. Mock control indicates the absence of added peptide. ‘*’ indicates P < 0.05 between indicated groups. (D) Product-ion spectrum (MS/MS) of the phosphorylated tryptic peptide corresponding to residues 399-414 (TQTPPVSPAPQPTEER) from immunoprecipitated cortactin in HUVECs treated with RSV. The asterisk indicates that an ion bears a phosphate group, and neutral loss of an H3PO4 is represented by ‘Δ’.