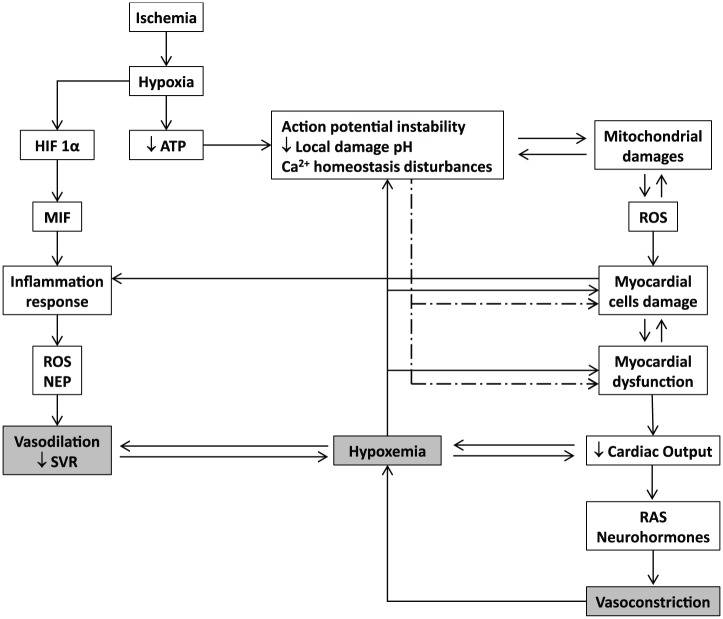
Figure 1.
Basic molecular pathophysiological cascade of acute myocardial infarction. Hypoxia triggers an acute failure in mitochondrial respiratory function when the diffusible oxygen stores become exhausted. Adenosine triphosphate reserves are rapidly depleted, and there is a respiratory shift toward an anaerobic profile. Lactate, H+ ions, CO2, and potassium accumulate may lead to arrhythmias, microendothelial damage, myocardiocytes stunning, and cell death. Adenosine triphosphate (ATP) depletion is irrevocably ligated to the inability of maintaining the normal negative resting membrane potential, to an alteration of calcium homeostasis (intracellular Ca2+ ([Ca2+]i) overload), which may eventually lead to different patterns of abnormal cardiac contraction. Mitochondrial functionality becomes abnormal, establishing the so-called “open pore” (mitochondrial permeability transition pore [mPTP]), leading to local cell death. In this scenario, mitochondria turn into an active ROS manufacturing plant that increases and perpetuates mitochondrial damages and dysfunction. The failure of myocardial contractility (contractility depression) is a precocious and multifactorial consequence of ischemia, which may eventually lead to reduced cardiac output and heart failure. This situation may translate into a self-perpetuated vicious circle, thus amplifying the ischemic episode and the myocardial wall stress. The local inflammatory reaction is a useful but critical operator within the myocardial ischemia/reperfusion damage process. Hypoxia itself activates the HIF-α/MIF axis and the consequent downstream inflammatory cascade. The locally secreted pro-inflammatory cytokines are involved in a self-perpetuating process in the ROS chain reaction, inflammation, and cellular damage.