Abstract
Background:
Research into the pathophysiology of bipolar disorder (BD) is limited by the inability to examine brain cellular processes in subjects with the illness.
Methods:
Endoscopic biopsy was performed in subjects with bipolar I disorder to establish olfactory neural progenitor (ONP) cell lines. Olfactory function was assessed prebiopsy and postbiopsy using the University of Pennsylvania Smell Identification Test (UPSIT). Cells were characterized to determine their lineage.
Results:
There were no significant complications associated with the biopsy procedure, including olfaction. Outpatient olfactory neuroepithelial biopsy yielded ONP cells in three out of 13 biopsy attempts (23.1%). ONPs were positive for neuron-specific proteins (β-tubulin III, nestin, hexaribonucleotide binding protein-3, and peripherin) and glia-specific proteins (glial fibrillary acidic protein and myelin basic protein).
Conclusions:
ONP cells can be obtained safely from awake outpatients and are potentially useful for pathophysiological studies of bipolar illness and perhaps other neuropsychiatric conditions. Such cells allow for the investigation of potential pathological cellular processes without the confounding factors of genetic manipulation, which is required for induced pluripotent cells.
Keywords: Progenitor cells, neural progenitors, olfactory neuroepithelium, bipolar disorder
Introduction
Study of the pathogenesis of bipolar disorder (BD) and other neuropsychiatric diseases is limited by the inability to directly examine brain cellular processes. Early work utilized freshly obtained red cells,1 platelets,2 lymphocytes,2,3 as well as immortalized lymphoblasts4 or fibroblasts.5 However, these cells have significant limitations. Platelets and red cells lack a nucleus and, therefore, cannot be used to examine gene response. Lymphocytes have limited life times in culture. Lymphoblasts and fibroblasts can be maintained in an indefinite culture but are generally incapable of expressing a wide variety of centrally expressed genes. Newer works have focused of pluripotent stem cells,6,7 but manipulations of these cells inducing an artificial stem cell-like state create a web of potential problems of data interpretations. For example, examination of processes of cellular death and resilience is very difficult in cells induced to become immortal.
Recently, several researchers have succeeded in isolating neural stem cells from adult brain.8 These cells can be obtained from central nervous system (CNS) in early postmortem samples9; however, this requires an elaborate system in which informed consent is given antemortem and brain tissue is accessed immediately after death. Alternatively, the olfactory system contains neuronal tissue, which is extracranial and continuously regenerated.10 The olfactory neuroepithelium (ONe) contains stem cells or neural progenitor cells that continually divide to produce both neurons and their supporting cells.10 Previous studies have established that olfactory neural progenitor (ONP) cells can be established from ONe biopsy both from 2 to 12 hours postmortem samples11 and anesthetized subjects undergoing sinus surgery.12 These cells have been used to examine the function of genes that may be important in BD.13
ONe biopsies have been used to establish time-limited primary biopsies from humans with BDs.14,15 These biopsies proved valuable in studying disease-specific physiological alterations such as calcium signaling14 and cell cycle.15 More recently, progenitor cells from the olfactory epithelium capable of differentiating into neural cells have been established from subjects with schizophrenia and BD. These cells have been found to express a lower amount of β-tubulin III in patients with BD than nonbipolar controls.16,17 Such findings hint that ONP is a promising cell model of neuropsychiatric disorders, in particular, for the development of patient-based, disease-relevant cellular models.
The current study was designed to demonstrate the feasibility of establishing ONP cells from ambulatory subjects with bipolar I disorder. ONP biopsies were performed on awake outpatients under local anesthesia. The study demonstrates that outpatient ONP biopsies are safe and well-tolerated by euthymic bipolar subjects and that immortal neuronal progenitor cells can be established to investigate the cellular physiology of this disorder.
Methods
Subjects
Thirteen subjects with bipolar I disorder were recruited. All subjects had to be currently euthymic as documented by clinical examination. All fulfilled diagnostic criteria for bipolar I disorder as defined by the Diagnostic and Statistics Manual fourth edition (DSM IV),18 documented with a Diagnostic Interview for Genetic Studies (DIGS).19,20 Lithium responsiveness was determined according to the Alda scale.21 Subjects with substance abuse disorders currently or within the previous six months were excluded. Subjects with active sinus disorder were also excluded. Subjects were reimbursed $500.00 to offset their time and effort. All provided informed consent. Human Subjects Protection Office approved the protocol.
Biopsy procedure
This procedure was performed in an office setting under local anesthesia. Prior to biopsy, subjects underwent comprehensive nasal endoscopy using a 0°, 4-mm rigid Hopkins rod endoscope to determine the preferred side to perform the procedure. Anesthesia was achieved with 2% pontocaine/0.5% phenylephrine hydrochloride solution sprayed into the nasal cavity supplemented with 1 mL of 1% xylocaine/1:100,000 epinephrine infiltrated into the dorsoposterior septum. Using microsurgical instruments, a 16-mm2 biopsy of mucosa with underlying periosteum was harvested from the nasal septum just anterior to the sphenoid rostrum (Figure 1). A small piece of gelfoam was placed over the biopsy site to complete the procedure. Endoscopic examination was performed two weeks and three months following the biopsy to monitor the complications.
Figure 1.
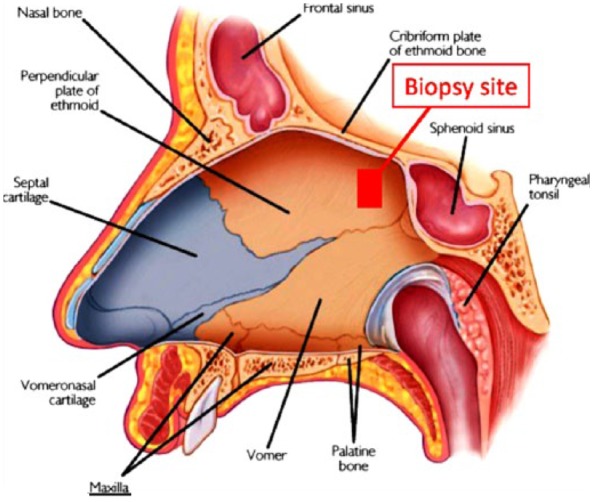
Site of olfactory neuroepithelial biopsy.
Olfactory testing
The University of Pennsylvania Smell Identification Test (UPSIT) (22 was used to assess olfaction in all subjects prior to the biopsy and three months after the procedure.
Culture procedures
The biopsy specimens were processed as previously described.11,12 The solid biopsy specimen was broken down with a solution of 0.05% trypsin (type XIII; Sigma-Aldrich) in Ca2+- and Mg2+-free Hank’s Balanced Salt Solution, and incubated with gently bubbling O2 and friction at 35°C for 90 minutes. The tissue was washed four times with 0.01% deoxyribonuclease (DNAaseI; Sigria) in Ca2+- and Mg2+-free complete Hank’s balanced salt solution. After centrifugation, the pellet was resuspended in progenitor cell culture medium (PCM) (1:1 mixture of Dulbecco’s modified Eagle medium [DMEM] and Ham’s F12 with N1 supplement and heparin 2 mg/mL; Gibco) with 0.7% ovomucoid, then centrifuged again, and re-suspended in PCM with 5% fetal bovine serum (Gibco). A total of 6–12 dishes from each biopsy were plated at 200 cells/cm2 with laminin–fibronectin-treated surfaces. The plate was treated with 0.5 mg/mL laminin (Cat # L2020; Sigma-Aldrich) and 1 mg/mL poly-l-ornithine (Cat # P4957; Sigma-Aldrich) 1:1 mixture for 45–60 minutes at 37°C. Approximately half of the medium was changed every three to four days. The cultures were routinely scanned for neurosphere clusters.
Differentiation
The differentiation was processed as previously described.23 The ONPs were adapted to the absence of serum via serial dilution of serum every two days for a week until the cells were finally cultured in DMEM/F12 supplemented with 1% B27 and 0.5% N2 and 100 μg/mL gentamycin (Gibco®, Thermo Fisher Scientific) for one week. Differentiation was achieved by treating ONPs for four days with retinoic acid (1 μM) and forskolin (5 μM), followed by three days in which the forskolin is replaced with sonic hedgehog (15 nM) in DMEM with 1% B27 and 0.5% N2. Vehicle controls excluded these compounds but used the solvent DMSO 0.2% (used to dissolve retinoic acid and forskolin) to the media.
Characterization of cells
Some characterization of ONP cells has been previously reported.24,25 For the current study, cell type-specific antigen proteins were examined by Western blot immunoassay. The neuronal markers (nestin, β-tubulin III, hexaribonucleotide binding protein-3 [NeuN], and peripherin) and glial markers (glial fibrillary acidic protein [GFAP], myelin basic protein [MBP], and Iba-1) were used to determine the level of lineage restriction.
Cells were lysed in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.4), 50 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM PMSF, and 1% protease inhibitor cocktail (Sigma-Aldrich) for 30 minutes followed by centrifugation at 4°C with 13,000 × g for 10 minutes, the supernatant was collected, and the protein concentrations were measured using the DC Protein Assay kits (Bio-Rad). Equal amount of cell lysis was used for each sample. The proteins were separated using the discontinuous sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) system with stacking gel (acrylamide 5%) and separating gel (acrylamide 8%–15%) based on the molecular weight of the target protein. After electrophoresis, the gels were electrotransferred onto Immobilon-P transfer membranes (Merck Millipore). The membranes were blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for one hour at 37°C followed by incubation at 4°C overnight with antinestin (1:500, AB5922; Merck Millipore), anti-β-tubulin III (1:500, T8660; Sigma-Aldrich), antiperipherin (1:1000, AB1530; Merck Millipore), anti-NeuN (1:300, MAB377; Merck Millipore), anti-GFAP (1:500, MAB360; Merck Millipore), anti-MBP (1:2000, AB980; Merck Millipore), anti-ionizing calcium-binding adaptor molecule 1 (Iba-1) (1:50, sc-98468; Santa Cruz Biotechnology), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:20,000 G9545; Sigma-Aldrich). The membranes were then washed with TBST followed by incubation with 1:3000 diluted peroxidase-conjugated goat-antirabbit or goat-antimouse IgG secondary antibody (Santa Cruz Biotechnology) at room temperature for one hour and detected by ECL Western blot detection reagents (Amersham Biosciences).
Results
Subjects
A total of 13 subjects underwent 14 biopsy procedures. (One subject underwent two biopsy procedures over six months apart.) All had bipolar I disorder. Only three samples resulted in permanent cultures (23.1%). The demographics of these samples are described in Table 1.
Table 1.
Bipolar subjects from whom samples were obtained.
| Subject | Gender | Age (yrs) | Medications at time of biopsy | DOI (yrs) | Mood State at time of biopsy | Li responsiveness |
|---|---|---|---|---|---|---|
| ONe111 | Male | 22 | Lithium | 5 | euthymic | Yes |
| ONe115 | Male | 47 | Divalproex | 22 | Dysthymic depression | Yes |
| ONe139 | Female | 56 | Divalproex | 34 | euthymic | Unknown |
DOI, duration of illness.
Olfactory function testing
The UPSIT was performed before and three months after the biopsy procedure. There was no significant difference in olfactory function (31.88 ± SD 2.75 before vs 32.0 ± 3.46 after, paired t = 0.13, P = 0.9; Figure 2).
Figure 2.
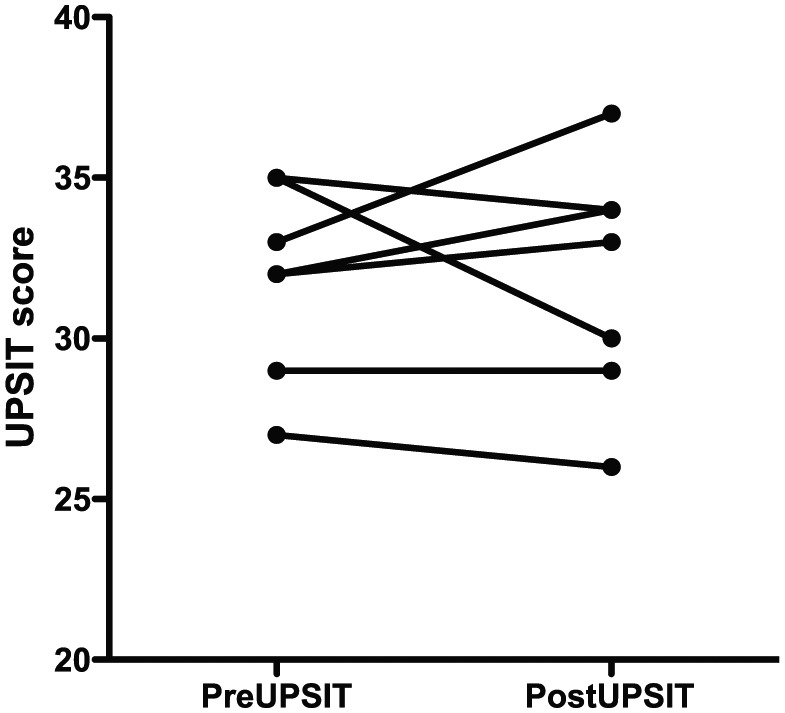
Scatter plot of UPSIT results in eight subjects undergoing biopsy procedure. Prebiopsy 32.0 ± SEM 1.04; postbiopsy 32.0 ± 1.23, paired t = 0.13, P = 0.9. Notes: two subjects had same values and are seen as one line on the graph; five patients did not perform postbiopsy testing and are not included.
Characterization of ONP cells
Three cell lines derived from bipolar I disorder subjects and seven cell lines from age- and sex-matched nonbipolar controls were used. ONP cells from bipolar subjects looked and behaved like ONP cells obtained previously from nonbipolar subjects.11,25 Bipolar-derived ONP cells were positive for the neuronal markers (nestin, β-tubulin III, NeuN, and peripherin) and the glial markers (GFAP and MBP), but Iba-1 was not detected. NeuN-66 kD and 48 kD were detected. MBP showed 21 kD, 18 kD, and 17 kD. None of the markers showed a difference between bipolar and nonbipolar cell lines (Table 2). These data are consistent with previous work with ONPs derived from nonbipolar controls.11,12,25 Immunocytochemical staining in these nonbipolar controls reveals a mixed population culture.11,12 Those ONPs were found to persist in culture for over two years without degradation of telomerase activity,24 a property believed to extend to the cells obtained from bipolar subjects.
Table 2.
Immunoblotting of neuronal markers (nestin, β-tubulin III, NeuN, and peripherin) and glial markers’ (GFAP and MBP) expressions in ONPs derived from bipolar and nonbipolar subjects.
| Nestin | β-tubulin III | NeuN | Peripherin | GFAP | MBP | |
|---|---|---|---|---|---|---|
| BD | 0.86 ± 0.15 | 1.20 ± 0.15 | 4.73 ± 0.25 | 1.19 ± 0.05 | 0.32 ± 0.02 | 2.51 ± 1.01 |
| Non-BD | 1.15 ± 0.24 | 1.23 ± 0.31 | 5.33 ± 1.16 | 0.92 ± 0.17 | 0.26 ± 0.04 | 3.78 ± 0.87 |
NeuN-66 kD and 48 kD both can be detected. MBP showed 21 kD, 18 kD, and 17 kD. The data present as total amounts of two bands of NeuN or three bands of MBP. Data expressed as the mean ± standard deviation of the ratio of density of target protein/GAPDH, three BD cell lines, and six non-BD cell lines. None of the proteins are statistically different in BD vs non-BD control by t-testing.
When cells are differentiated, the expression of β-tubulin, nestin, and NeuN does not change, but peripherin, a marker of mature neurons, increases in both bipolar and non-bipolar cells. Interestingly, GFAP, a marker for astrocytes, also increases in both bipolar and nonbipolar cells. MBP, an oligodendrocyte marker, and Iba-1, a microglial marker, do not change compared to undifferentiated cells.
Sensitivity to proapoptotic stimuli
ONPs derived from bipolar subjects exhibited a greater sensitivity to proapoptotic stimuli. Glutamate 0.1 M increased apoptosis in a dose- and time-dependent manner. Cells obtained from bipolar subjects exhibit greater apoptosis than cells obtained from matched control subjects (Figure 3). However, the power of the current sample size (n = 6) is quite small at 0.13.
Figure 3.
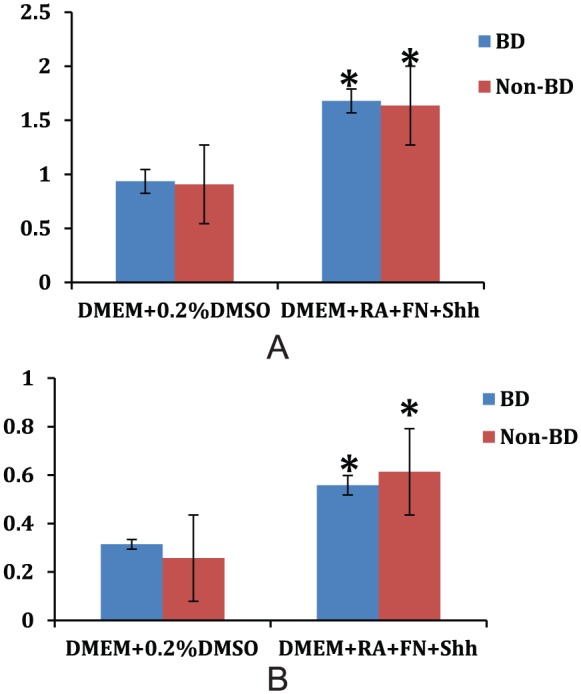
Peripherin (A) and GFAP (B) expressions increase after differentiation in olfactory neuroepithelial progenitors. There is no difference between bipolar and nonbipolar cell lines (*P < 0.05, compared to undifferentiated cell).
Discussion
ONP cells can be obtained safely from awake, euthymic bipolar outpatients by endoscopic biopsy (Figure 1). This procedure does not affect the olfactory function (Figure 2).
ONP cells spontaneously differentiated in culture and showed the heterogeneity of the cell types. This heterogeneity has been shown previously with immunocytochemistry11,12 and, in this study, with the more sensitive Western blot method. Utilizing Western blot, the astrocyte marker, GFAP, could be detected. When differentiation is induced with a combination of retinoic acid, forskolin, and sonic hedgehog, the expression of markers of mature neurons and astrocytes increased, suggesting that this form of differentiation maintains the heterogeneous nature of the cultures. Prior to induced differentiation, ONPs express both mature and immature neuronal and glial markers. Differentiation of ONPs into cell type-specific neurons has been previously demonstrated with the introduction of specific maturation genes.23,26
Intracellular processes are important aspects of the pathophysiology of BD. These include identification of genes that play an important role in the regulation of the intracellular ionic environment (eg, ANK3, TRPM2, and KCNQ227–30) with nearly 75% of all potential genes being involved in ion regulation31; especially, intracellular sodium32,33 and calcium2 concentrations are elevated in bipolar subjects. The availability of olfactory neuroepithial progenitor cells obtained from bipolar subjects and nonbipolar controls allows for the examination of ionic transport and other cellular processes that cannot be examined in whole patients or by noninvasive techniques. A recent example is the use of these ONPs to examine gene expression during apoptosis.34 Specifically, we have demonstrated that ONPs with the genetic heritage of bipolar I disorder were more sensitive to glutamate-induced apoptosis. Under expression of the BRAF gene and protein may contribute to this increased sensitivity to apoptosis of cells obtained from bipolar subjects.34
While primary culture of the ONe has proven useful in the study of the pathophysiology of neuropsychiatric diseases including BD,14,15 other laboratories have succeeded in isolating progenitor cells that produce permanent cultures for ongoing study without repeated biopsies (Warsh, unpublished).16 Benítez-King et al16 utilized a special brush to create exfoliative debris of the ONe and cultured that debris. They did not search for neurospheres but were able to detect neural-type cells within two weeks of culture. They did not report their success rate in obtaining permanent cell lines. We utilized a biopsy of solid tissue, which was dissociated in culture, and ultimately yielded neurospheres, which produced the ONP cells. Our success rate was 23.1%. The culture conditions were essentially equivalent. Similarly, the cellular characterization appears very similar.16,17 One possible exception is the reduced expression of β-tubulin in bipolar patients from Mexico17 but not in our sample (Table 2). However, this may be related to the fact that in those studies, cytosolic and membrane-cytoskeletal components were fractionated and separated, whereas we utilized whole cell homogenates. Reproducing these results with a larger sample size will allow for a more definitive conclusion to be drawn.
The other major approach to examine neural activity at the cellular level has been pioneered utilizing induced pluripotent stem cells (iPSCs).35 These are created by obtaining cells via a biopsy (most often fibroblasts from a skin biopsy) and infecting them with genes that convert the terminally mature cell back to a stem cell.7,35 The newly created stem cells can then be redifferentiated into mature neurons or glia (or any other cell type) utilizing an appropriate combination of transfected genes.35,36 Although this approach is reasonable, the conversion of terminally differentiated cells back into pluripotent cells may induce epigenetic changes that may modify the outcome of subsequent experiments in an unknown and unpredictable fashion. Although this is only a theoretical concern, it casts doubt on work with iPSCs that cannot readily be addressed. Nonetheless, these cells have been shown to be useful models in the examination of ionic cellular processes.35
There are clear limitations to this approach. Foremost is the perceived invasiveness of the procedure. The only other similar methodology is the use of induced pluripotent cell lines. These lines are usually obtained from skin biopsies. Given the lack of effect on olfactory function, the relative invasiveness of the procedures is similar. Expansion of the number of cells available will allow detailed examination of neural processes. The apparent low success rate is reflective of the fact that it is difficult to identify ONe visually, and in most people, it is present in patches that are interdispersed with nonolfactory epithelium. Consequently, the biopsy may occasionally miss the proper area. Clearly, additional work is required. In addition, although these cells can be studied in a multitude of ways to determine the cellular response to physiological or environmental factors, they cannot provide direct information on what those factors are. In other words, these cells can be used to test hypotheses regarding the pathophysiological changes in bipolar illness but not to generate them. Finally, the heterogeneous nature of the cultures is somewhat problematic. It should be remembered that even when iPSCs are induced to differentiate, the resulting culture is heterogenous.35 However, neuronal cultures require glial cells to maintain their health; consequently, data obtained utilizing heterogeneous cultures may actually be more applicable than homogenous cultures, since homogenous cultures are actually not reflective of healthy neuronal cells. Nonetheless, if experiments require purified cultures, it may be possible to isolate specific cell populations with immunomagnetic separation, a procedure that uses ferrous beads coated with antibodies that will bind to antigens present on the surface of cells.
Despite these limitations, the use of ONP cells to examine the pathophysiology of bipolar illness at the cellular level is a novel and potentially powerful approach. These cells are in some respects superior to the more commonly used iPSCs, since they are genetically unaltered. The current study demonstrates the feasibility of obtaining these biopsy specimens from awake ambulatory subjects and documents that the basic characteristics of these cells are similar to those obtained from non-bipolar control subjects. In addition, preliminary data suggest that there may be important differences in sensitivity to glutamate-induced excitotoxicity. Continued work with these cells should enlighten the scientific community regarding the pathophysiology of BD.
Footnotes
Peer Review:6 peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1263 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Brain and Behavior Research Foundation (NARSAD) to RSE-M. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
Declaration of Conflicting Interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs. CL and FJR have a financial interest in RhinoCyte™ Inc., which owns the cells. However, mechanisms have been put into places that prevent them from directly benefiting from this work. Dr. RSE receives research funding from the National Institutes of Health, the state of Kentucky, Merck, Alexza, Janssen, and Psychnostics; and he is also a speaker for Allergan, Lundbeck, Merck, Otsuka, Sunovion, and Takeda. None of the other others have relevant conflicts of interest.
Abbreviations: CNS, central nervous system; DIGS, Diagnostic Interview for Genetic Studies; DMEM, Dulbecco’s modified Eagle medium; DSM IV, Diagnostic and Statistics Manual 4th edition; GFAP, glial fibrillary acidic protein; iPSC, induced pluripotent stem cells; MBP, myelin basic protein; NeuN, hexaribonucleotide binding protein-3; ONe, olfactory neuroepithelium; ONP, olfactory neural progenitor; PCM, progenitor cell culture medium; TBST, Tris-buffered saline containing 0.1% Tween 20; UPSIT, University of Pennsylvania Smell Identification Test
Author contributions: Conceived and designed the experiments: RSE and YG. Analyzed the data: YG and RSE. Obtained the cell lines: WW, CL, FJR. Wrote the first draft of the manuscript: RSE. Contributed to writing the manuscript: all coauthors. Agree with manuscript results and conclusions: all coauthors. Jointly developed the structure and arguments for the paper: YL and RSE. Made critical revisions and approved final version: all coauthors.
References
- 1. Naylor GJ, McNamee HB, Moody JP. Changes in erythrocyte sodium and potassium on recovery from a depressive illness. Br J Psychiatry. 1971;118:219–223. [DOI] [PubMed] [Google Scholar]
- 2. Dubovsky SL, Murphy J, Thomas M, Rademacher J. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry. 1992;149(1):118–120. [DOI] [PubMed] [Google Scholar]
- 3. El-Mallakh RS, Li R, Worth CA, Peiper SC. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41:33–37. [DOI] [PubMed] [Google Scholar]
- 4. Buss TJ, Li R, Peiper SC, El-Mallakh RS. Lymphoblastoid transmembrane potential in bipolar patients, their siblings, and unrelated healthy comparison subjects. Psychiatry Res. 1996;59(3):197–201. [DOI] [PubMed] [Google Scholar]
- 5. Cataldo AM, McPhie DL, Lange NT, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177(2):575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin M, Pedrosa E, Shah A, et al. RNA-Seq of human neurons derived from iPS cells reveals candidate long non-coding RNAs involved in neurogenesis and neuropsychiatric disorders. PLoS One. 2011;6(9):e23356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madison JM, Zhou F, Nigam A, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20(6):703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Germain ND, Banda EC, Becker S, Naegele JR, Grabel LB. Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells. Stem Cells Dev. 2013;22(10):1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall CT, Lu C, Winstead W, et al. The therapeutic potential of human olfactory-derived stem cells. Histol Histopathol. 2006;21:633–664. [DOI] [PubMed] [Google Scholar]
- 10. Calof AI, Mumm JS, Rim PC, Shou J. The neuronal stem cells of the olfactory epithelium. J Neurobiol. 1998;36:190–205. [DOI] [PubMed] [Google Scholar]
- 11. Roisen FJ, Klueber KM, Lu CL, et al. Adult human olfactory stem cells. Brain Res. 2001;890:11–22. [DOI] [PubMed] [Google Scholar]
- 12. Winstead W, Marshall CT, Lu CL, Klueber KM, Roisen FJ. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol. 2005;19:83–90. [PubMed] [Google Scholar]
- 13. Gao Y, Lei Z, Lu C, Roisen FJ, El-Mallakh RS. Effect of ionic stress on apoptosis and the expression of TRPM2 in human olfactory neuroepithelial-derived progenitors. World J Biol Psychiatry. 2010;11(8):972–984. [DOI] [PubMed] [Google Scholar]
- 14. Hahn CG, Gomez G, Restrepo D, et al. Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry. 2005;162(3):616–618. [DOI] [PubMed] [Google Scholar]
- 15. McCurdy RD, Féron F, Perry C, et al. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82(2-3):163–173. [DOI] [PubMed] [Google Scholar]
- 16. Benítez-King G, Riquelme A, Ortíz-López L, et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J Neurosci Methods. 2011;201(1):35–45. [DOI] [PubMed] [Google Scholar]
- 17. Solís-Chagoyán H, Calixto E, Figueroa A, et al. Microtubule organization and L-type voltage-activated calcium current in olfactory neuronal cells obtained from patients with schizophrenia and bipolar disorder. Schizophr Res. 2013;143(2-3):384–389. [DOI] [PubMed] [Google Scholar]
- 18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 19. Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859; Discussion 863–864. [DOI] [PubMed] [Google Scholar]
- 20. Faraone SV, Blehar M, Pepple J, et al. Diagnostic accuracy and confusability analyses: an application to the diagnostic interview for genetic studies. Psychol Med. 1996;26(2):401–410. [DOI] [PubMed] [Google Scholar]
- 21. Tighe SK, Ritchey M, Schweizer B, et al. Test-retest reliability of a new questionnaire for the retrospective assessment of long-term lithium use in bipolar disorder. J Affect Disord. 2015;174:589–593. [DOI] [PubMed] [Google Scholar]
- 22. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 pt 1):176–178. [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Klueber KM, Guo Z, et al. Induction of neuronal differentiation of adult human olfactory neuroepithelial-derived progenitors. Brain Res. 2006;1073–1074:109-119. [DOI] [PubMed] [Google Scholar]
- 24. Marshall CT, Guo Z, Lu C, et al. Human adult olfactory neuroepithelial derived progenitors retain telomerase activity and lack apoptotic activity. Brain Res. 2005;1045:45–56. [DOI] [PubMed] [Google Scholar]
- 25. Othman M, Lu C, Klueber K, Winstead W, Roisen FJ. Clonal analysis of adult human olfactory neurosphere forming cells. Biotechnic Histochem. 2005;80(5-6):189–200. [DOI] [PubMed] [Google Scholar]
- 26. Wang M, Lu C, Roisen F. Adult human olfactory epithelial-derived progenitors: a potential autologous source for cell-based treatment for Parkinson’s disease. Stem Cells Transl Med. 2012;1(6):492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dick DM, Foroud T, Flury L, et al. Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the national institute of mental health genetics initiative. Am J Hum Genet. 2003;73:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu C, Macciardi F, Li PP, et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(1):36–43. [DOI] [PubMed] [Google Scholar]
- 29. Ferreira MA, O’Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40(9):1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith EN, Bloss CS, Badner JA, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Askland K. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94:35–66. [DOI] [PubMed] [Google Scholar]
- 32. Coppen A, Shaw DM, Malleson A, Costain R. Mineral metabolism in mania. Br Med J. 1966;1:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El-Mallakh RS, Wyatt RJ. The Na,K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37:235–244. [DOI] [PubMed] [Google Scholar]
- 34. Schroeder E, Gao Y, Lei Z, Roisen F, El-Mallakh RS. The gene BRAF is underexpressed in bipolar subject olfactory neuroepithelial progenitor cells undergoing apoptosis. Psychiatry Res. 2016;236:130–135. [DOI] [PubMed] [Google Scholar]
- 35. Mertens J, Wang QW, Kim Y, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527(7576):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2015;73:63–83. [DOI] [PubMed] [Google Scholar]


