Abstract
Objective
In pediatric ARDS, lung injury is mediated by immune activation and severe inflammation. Therefore, we hypothesized that patients with elevated pro- and anti-inflammatory cytokines would have higher mortality rates and that these biomarkers could improve risk-stratification of poor outcomes.
Design
Multicenter prospective observational study.
Setting
We enrolled patients from 5 academic PICUs between 2008–2015.
Patients
Patients were 1 month to 18 years old, used noninvasive or invasive ventilation, and met the American European Consensus Conference definition of ARDS.
Interventions
None.
Methods
Eight pro-inflammatory and anti-inflammatory cytokines were measured on ARDS day 1 and correlated with mortality, ICU morbidity as measured by survivor PELOD score, and biomarkers of endothelial injury, including angiopoietin-2, von Willebrand Factor, and soluble thrombomodulin.
Measurements & Main Results
We measured biomarker levels in 194 patients, including 38 ARDS nonsurvivors. IL-6, IL-8, IL-10, IL-18, and TNF-R2 were each strongly associated with all-cause mortality, multiple markers of ICU morbidity, and endothelial injury. A multiple logistic regression model incorporating OI, IL-8, and TNF-R2 was superior to a model of OI alone in predicting the composite outcome of mortality or severe morbidity (AUROC 0.77 [0.70–0.83] vs. 0.70 [0.62–0.77], p=0.042).
Conclusions
In pediatric ARDS, pro- and anti-inflammatory cytokines are strongly associated with mortality, ICU morbidity, and biochemical evidence of endothelial injury. These cytokines significantly improve the ability of the OI to discriminate risk of mortality or severe morbidity and may allow for identification and enrollment of high-risk subgroups for future studies.
Keywords: Intensive care units, cytokines, acute lung injury, hematopoietic stem cell transplantation, interleukins
INTRODUCTION
Pediatric acute respiratory distress syndrome (ARDS) is a clinically heterogeneous disorder characterized by injury to the alveolar-capillary interface, resulting in significant impairment of oxygenation (1, 2). This syndrome is associated with 15–20% mortality as well as substantial morbidity among survivors (3, 4), yet imprecision in identifying high-risk patients early in their course limits the application of experimental therapies that might further improve outcomes. While triggering events include both local and systemic insults, a common pathway is the resultant inflammatory cascade that mediates both pulmonary endothelial and alveolar epithelial damage (5, 6).
A principal component of this inflammatory cascade is the release of chemokines such as IL-8 and macrophage inflammatory protein 1β (MIP-1β) by local antigen presenting cells and endothelial cells, leading to migration of activated neutrophils to the lungs (7, 8) and subsequent release of cytokines such as IL-6 and TNF-α (9, 10). In concert, these mechanisms lead to an increase in lung endothelial permeability and ongoing immune activation, which can further propagate the cycle of cell damage (11, 12). We have previously identified evidence of this cell damage in pediatric ARDS patients by correlating mortality with circulating markers of endothelial cell injury, including angiopoietin-2, von Willebrand Factor, and soluble thrombomodulin (13, 14). To date, however, little direct evidence exists linking systemic immune activation with pediatric ARDS outcomes or markers of endothelial damage.
We therefore undertook this study to correlate circulating pro- and anti-inflammatory cytokine levels in pediatric ARDS patients with mortality, morbidity, and biomarkers of endothelial injury. We used a commercially available multiplex panel of 21 cytokines related to macrophage, neutrophil, T-cell, and B-cell activation (Supplemental Figure 1), retained only those reliably detected above the lower limit of quantitation (LLOQ), and then analyzed the performance characteristics of these biomarkers according to consensus guidelines for biomarker development (15–18). We hypothesized that elevated cytokine levels would correlate with clinical outcomes and markers of endothelial damage in pediatric ARDS and might augment the prognostic utility of the OI in predicting mortality and morbidity.
METHODS
Patients
Daily screening occurred at five PICUs between 2008–2015. Inclusion criteria were (1) 30 days < age ≤18 years, (3) respiratory support of either CPAP, BiPAP, or invasive mechanical ventilation, and (3) ARDS or acute lung injury as defined by the American European Consensus Conference (19). Exclusion criteria were corrected gestational age <36 weeks and limitation of care precluding endotracheal intubation. This study was approved by the Institutional Review Board at each center with consent obtained from each patient/surrogate.
Measurements
All cytokine measurements were carried out on plasma samples collected within 24 hours of onset of ARDS (hereafter referred to as ARDS day 1). As daily pediatric phlebotomy restrictions limited available blood volume for research, we used a multiplex immunoassay to measure IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6 IL-7, IL-8, IL10, IL-12, IL-15, IL-17, IL-18, IL-23, MIP-1α, MIP-1β, TNF-α, and TNF-R2 and an Ultrasensitive Immunoassay to repeat measurement of TNF-α (Myriad RBM, Austin, TX, USA; Supplemental Content). Only cytokines measured above the lower limit of quantitation (LLOQ) in ≥75% of patients were included in the analysis.
Primary Outcomes
We measured associations between cytokines and two primary outcomes: (1) all-cause hospital mortality and (2) survivor morbidity, estimated by the highest survivor Pediatric Logistic Organ Dysfunction score (PELOD) of measurements taken on days 1–7, 14, 21, and 28 (20). To optimize statistical power for multivariable regression, we combined these two outcomes into a composite outcome of either mortality or severe morbidity, defined as the top quartile of survivor PELOD score.
Secondary Outcomes
We measured associations between cytokines and day 1 ARDS severity, estimated by the PaO2/FiO2 ratio (P/F ratio), the oxygenation index (OI), and the Pediatric Risk of Mortality-3 score (PRISM-3), the latter solely in cases where ARDS day 1 coincided with PICU day 0 or 1 (Supplemental Content) (21). When an arterial line was not present, PaO2 was imputed from SpO2 ≤97% according to a log-linear equation (22). Finally, we measured associations between day 1 cytokines and three biomarkers of endothelial injury (Ang-2, vWF, and sTM) (13, 14).
Confounding Variables
To account for underlying variation in cytokine levels due to demographic diversity, we adjusted for age, sex, and patient-reported race (23–25). We also tested for relationships with a history of hematopoietic cellular transplantation (HCT), which we have previously shown to significantly impact pediatric ARDS outcomes and plasma biomarkers (26–28).
Statistics
Selection of variables for logistic regression models used the least angle regression methodology, which employs forward stagewise selection to identify and rank regression variables according to their correlation with outcome, and then maximizes parsimony by identifying among equally robust models the model with the least prediction error (29). Multivariable models were validated using 5-fold internal cross-validation, which minimizes overfitting by deriving the model in a randomly-selected 90% of subjects then repeating the process 5 times and averaging the results (Supplemental Content) (30).
RESULTS
Patient Characteristics and Outcomes
Of 284 enrolled patients, 194 had adequate plasma available for cytokine measurements and were included in this study (Supplemental Table 1). Of these 194, 107 were male (55%), 135 were Caucasian (70%), and the median age was 4.9 years (IQR 0.9–11.5, Table 1). Patients were admitted to the PICU a median of one day before ARDS onset (IQR 0–2) and on the first day after ARDS onset (ARDS day 1), the median P/F ratio was 136.5 (IQR 90.1–218), the median OI was 9.4 (IQR 5.3–18.3), and 176 patients received conventional mechanical ventilation (91%). All-cause hospital mortality occurred in 38 patients (20%) and median survivor PELOD score and ICU LOS were 20 (IQR 11–30) and 12 days (IQR 8–19).
Table 1. Characteristics of Enrolled Patients with Biomarker Measurements.
Legend: Associations tested with Fisher exact test for categorical variables and Wilcoxon rank sum for non-normally distributed continuous variables. P/F ratio n=193 (1 patient excluded due to no arterial line and SpO2>97%), OI n=191 (1 patient excluded due to no arterial line and SpO2>97%; 2 patients excluded due to missing MAP), PRISM-3 n=126 (48 patients excluded due to PRISM-3 calculated >1 day before ARDS diagnosis; 20 patients excluded due to majority of PRISM-3 components not measured).
| Characteristics | All patients (n=194) | Non-Survivors (n=38) | Survivors (n=156) | Significance |
|---|---|---|---|---|
|
| ||||
| Age (median years, IQR) | 4.9 (0.9–11.5) | 7.7 (2.5–12.6) | 3.8 (0.8–11.4) | p=0.186 |
|
| ||||
| Sex (male n, %) | 107 (55.2) | 25 (65.8) | 82 (52.6) | p=0.151 |
|
| ||||
| Race (n, %) | p=0.630 | |||
| White | 135 (69.6) | 25 (65.8) | 110 (70.5) | |
| Unknown | 22 (11.3) | 6 (15.8) | 16 (10.3) | |
| Black | 14 (7.2) | 2 (5.3) | 12 (7.7) | |
| Asian/PI | 10 (5.2) | 1 (2.6) | 9 (5.8) | |
| Multiple | 12 (6.2) | 4 (10.5) | 8 (5.1) | |
| American Indian | 1 (0.5) | 0 (0) | 1 (0.6) | |
|
| ||||
| Lung Injury Etiology (n, %) | p=0.619 | |||
| Pneumonia | 109 (56.2) | 22 (57.9) | 87 (55.8) | |
| Sepsis | 42 (21.7) | 9 (23.7) | 33 (21.2) | |
| Other | 16 (8.3) | 1 (2.6) | 15 (9.6) | |
| Trauma | 12 (6.2) | 2 (5.3) | 10 (6.4) | |
| Aspiration | 10 (5.2) | 2 (5.3) | 8 (5.1) | |
| TRALI | 5 (2.6) | 2 (5.3) | 3 (1.9) | |
|
| ||||
| Infectious Trigger (n, %) | 151 (77.8) | 31 (81.6) | 120 (76.9) | p=0.665 |
|
| ||||
| HCT (n, %) | 21 (10.8) | 13 (34.2) | 8 (5.1) | p<0.001 |
|
| ||||
| Days in PICU Prior to ARDS Onset (median, IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | p=0.749 |
|
| ||||
| Day 1 Oxygenation Metric (n, %) | p=0.180 | |||
| Arterial Line (PaO2) | 168 (86.6) | 30 (79.0) | 138 (88.5) | |
| Pulse Oximeter (SpO2) | 26 (13.4) | 8 (21.0) | 18 (11.5) | |
|
| ||||
| Day 1 Respiratory Support (n, %) | p=0.808 | |||
| Noninvasive (CPAP/BiPAP) | 9 (4.6) | 2 (5.3) | 7 (4.5) | |
| Invasive (Conventional) | 176 (90.7) | 34 (89.5) | 142 (91.0) | |
| Invasive (HFOV) | 9 (4.6) | 2 (5.3) | 7 (4.5) | |
|
| ||||
| Day 1 Illness Severity (median, IQR) | ||||
| PaO2/FiO2 Ratio (P/F) | 136.5 (90.1–218) | 103.8 (85.7–161.7) | 146.7 (90.2–226.7) | p=0.027 |
| Oxygenation Index (OI) | 9.4 (5.3–18.3) | 13.4 (7.8–25.4) | 8.0 (5.0–16.9) | p=0.004 |
| PRISM-3 | 13 (8–21) | 18 (12.5–21.5) | 13 (7–20) | p=0.009 |
Elevations in Inflammatory Cytokines Are Strongly Associated with Mortality
Five pro-inflammatory cytokines (IL-6, IL-8, IL-18, MIP-1β, TNF-α) and three anti-inflammatory cytokines (IL-1RA, IL-10, and TNF-R2) were measured above the LLOQ in ≥75% of patients and were included in subsequent analyses. Univariate Analysis: When stratified by mortality, non-survivors had significantly higher levels of IL-6, IL-8, IL-18, IL-10, and TNF-R2 than survivors (Figure 1, Table 2). The AUROCs for discriminating mortality for the 8 cytokines ranged from 0.53–0.68 and was highest for IL-8 (0.68, 95% CI 0.60–0.77); none were significantly different than that of the OI (0.70, 95% CI 0.62–0.77, Supplemental Table 2). Adjusted Analysis: In order to account for potential confounders to these relationships, we adjusted for age, sex, race, OI, and history of HCT, the latter being associated with significantly higher levels of multiple inflammatory markers including IL-8, IL-18, and TNF-R2 (Supplemental Tables 3). After adjustment, elevated IL-6 and IL-8 remained significantly associated with increased odds of mortality (Supplemental Table 4). All 8 assessed biomarkers were each positively correlated with the OI and PRISM-3 sore, and IL-6, IL-8, IL-18, IL-1RA, IL-10, and TNF-R2 were each negatively correlated with the P/F ratio (Supplemental Table 5, Supplemental Figure 2).
Figure 1. Pro- and Anti-Inflammatory Cytokine Distributions in Pediatric ARDS.
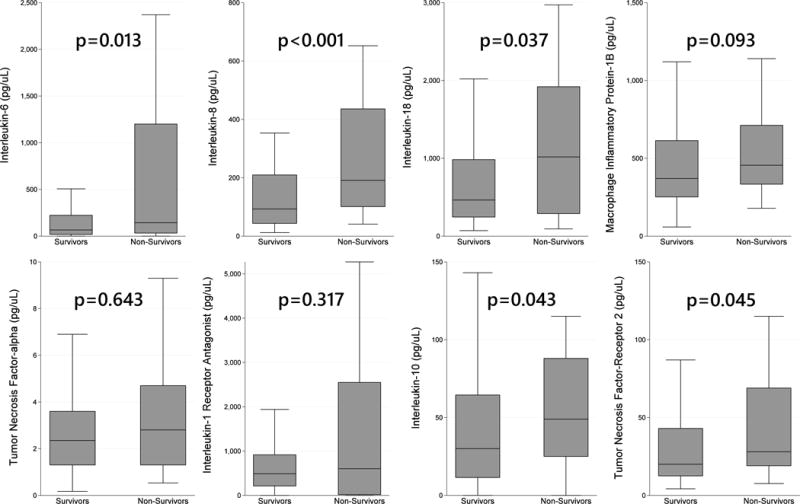
Legend: Distributions of pro-inflammatory (IL-6, IL-8, IL-18, MIP-1β, TNF-α) and anti-inflammatory cytokines (IL-1RA, IL-10, TNF-R2). IL-6, IL-8, IL-18, IL-10, and TNF-R2 were all greater in nonsurvivors than survivors. Upper (75th percentile + 1.5 IQR) and lower (25th percentile – 1.5 IQR) outliers not depicted.
Table 2. Plasma Levels of Cytokines, Stratified by Mortality.
Legend: Cytokine levels expressed as median pg/mL with IQR. Association tested with Wilcoxon rank sum test. n=194 for all measurements except n=151 for TNF-α.
| Cytokines | All patients (n=194) | Non-Survivors (n=38) | Survivors (n=156) | Significance | |
|---|---|---|---|---|---|
| Pro- | IL-6 | 74 (24–264.5) | 144 (33–1200) | 66 (18–223) | p=0.013 |
| IL-8 | 116 (51–236) | 191 (101–436) | 93 (43.5–209.5) | p<0.001 | |
| IL-18 | 490 (256–1120) | 1017.5 (289–1920) | 464 (245–982.5) | p=0.037 | |
| MIP-1β | 390.5 (266–630) | 455 (334–712) | 370.5 (251.5–613.5) | p=0.093 | |
| TNF-α | 2.4 (1.3–3.9) | 2.8 (1.3–4.7) | 2.4 (1.3–3.6) | p=0.643 | |
| Anti- | IL-1RA | 544 (186–950) | 600.5 (17–2550) | 488 (213–915.5) | p=0.317 |
| IL-10 | 34 (12–71) | 49 (25–88) | 30 (11.5–64.5) | p=0.043 | |
| TNF-R2 | 22 (13–47) | 28 (19–69) | 20 (12.5–43) | p=0.045 | |
Elevations in Inflammatory Cytokines Are Associated with Survivor ICU Morbidity
To assess morbidity in survivors, we examined the relationships between day 1 cytokines, survivor PELOD score, and ICU LOS. Day 1 IL-6, IL-8, IL-18, MIP-1β, TNF-α, IL-10, and TNF-R2 were each strongly associated with elevated PELOD score after adjustment for age, sex, and race (Supplemental Table 6). The strongest correlation was observed between PELOD and TNF-R2 (ρ=0.430, p<0.001). Increasing quartile of survivor PELOD was significantly associated with greater levels of all cytokines (Supplemental Figure 3). Elevated MIP-1β and TNF-R2 were weakly associated with increased survivor ICU LOS.
Elevations in Inflammatory Cytokines Are Associated with Biomarkers of Endothelial Injury
In order to associate cytokines with biochemical evidence of endothelial injury, we measured correlations between cytokines and plasma Ang-2, vWF, and sTM. IL-6, IL-8, TNF-α, IL-10, and TNFR-2 were each positively correlated with Ang-2, and IL-8, IL-1RA, IL-10, and TNF-R2 were each positively correlated with sTM (Supplemental Table 7). The strongest correlations were observed between IL-6 and Ang-2 (ρ=0.463, p<0.001) and between IL-8 and Ang-2 (ρ=0.443, p<0.001).
Elevations in Inflammatory Cytokines Help Distinguish Risk of Mortality or Severe Morbidity
Given this evidence, we used the least angle regression methodology to select variables for a regression model that would improve risk-stratification of the OI ratio by incorporating biomarker levels. To maximize statistical power, we combined our two primary outcomes into a composite outcome of either mortality or severe morbidity, defined as the top quartile of survivor PELOD score (PELOD ≥30). The model with maximum parsimony and minimum prediction error included the OI, IL-8, and TNF-R2 and predicted the composite outcome with a 5-fold internally cross-validated AUROC of 0.77 (95% CI 0.70–0.83). Compared to the OI alone (AUROC 0.70, 95% CI 0.62–0.77), this model had significantly greater fit (likelihood ratio test p<0.001) and AUROC (p=0.042, Figure 2).
Figure 2. Receiver Operating Characteristics for Mortality or Severe Morbidity in Pediatric ARDS.
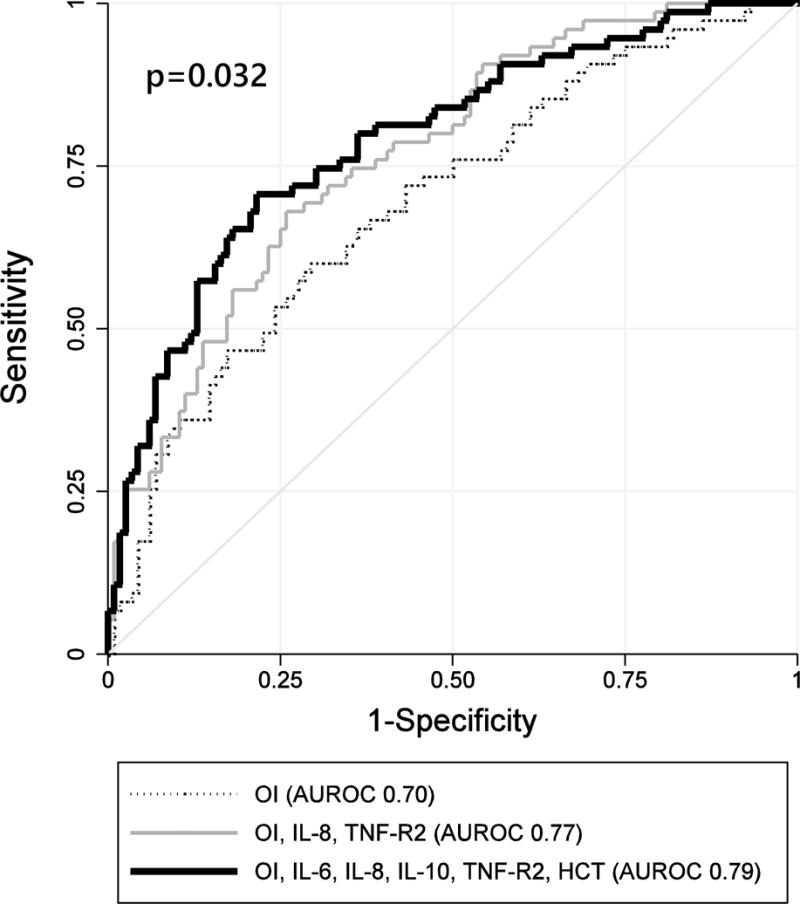
Legend: The AUROC for the OI ratio (dotted line) was 0.70 (95% CI 0.62–0.77). The AUROC for the model including the OI, IL-8, and TNF-R2 (gray line) was of 0.77 (95% CI 0.70–0.83). The AUROC for the expanded model including the OI, IL-6, IL-8, IL-10, TNF-R2, and HCT history (black line) was of 0.79 (95% CI 0.72–0.86). Chi-squared test for difference in ROC curves p=0.032.
We then used the least angle regression methodology to test an expanded set of variables including the OI, HCT status, any of the 8 measured cytokines, and any of the 3 biomarkers of endothelial injury. The expanded model with maximum parsimony and minimum prediction error included the OI, HCT status, IL-6, IL-8, IL-10, and TNF-R2, predicted mortality or severe morbidity with an internally cross-validated AUROC of 0.79 (95% CI 0.72–0.86) and was well-calibrated over each quartile (Hosmer-Lemeshow goodness-of-fit p=0.903, Supplemental Table 8). Compared to the OI alone, this expanded model had significantly greater fit (likelihood ratio test p<0.001) and AUROC (p=0.011).
When we divided patients into quartiles of risk according to the expanded model, we found that 74% of those in the highest quartile of risk died or had severe morbidity (sensitivity 74%, specificity 72%, likelihood ratio 2.7 [95% CI 2.0–3.7]). This was significantly greater than the 64% rate of death or severe morbidity for those in the top quartile of risk according to the OI alone (sensitivity 64%, specificity 69%; likelihood ratio 2.0 [95% CI 1.5–2.8], McNemar’s p=0.025). The majority of patients who reached the outcome of death or severe morbidity did so in the first 2 weeks of ICU stay (Supplemental Figure 4).
Elevations in Inflammatory Cytokines Improve PALICC Risk-Stratification
The next goal was to assess whether risk-stratification according to Pediatric Acute Lung Injury Consensus Conference (PALICC) defined ARDS severity could be improved by the addition of cytokine levels (31). ARDS severity on day 1 was strongly associated with both mortality (10% for mild [9/86], 21% for moderate [10/47], and 31% for severe [19/61], p=0.007) and the composite outcome of mortality or severe morbidity (24% for mild [21/86], 40% for moderate [19/47], and 61% for severe [37/61], p<0.001). For each of the 3 ARDS severities, we then further stratified patients according to whether their day 1 IL-8 level was above or below the cohort median (116 pg/uL). In both the moderate ARDS group and the severe ARDS group, patients with day 1 IL-8 levels above the cohort median had significantly greater rates of mortality or severe morbidity than patients with day 1 IL-8 levels below the cohort median (57% [16/28] vs. 16% [3/19], p=0.005 among those with moderate ARDS; and 70% [28/40] vs. 43% [9/21], p=0.039, among those with severe ARDS; Figure 3). We found similar results in analogous comparisons using IL-10 and TNF-R2 (Supplemental Table 9).
Figure 3. Kaplan Meier Function for Mortality or Severe Morbidity According to PALICC ARDS Severity and IL-8 Level.
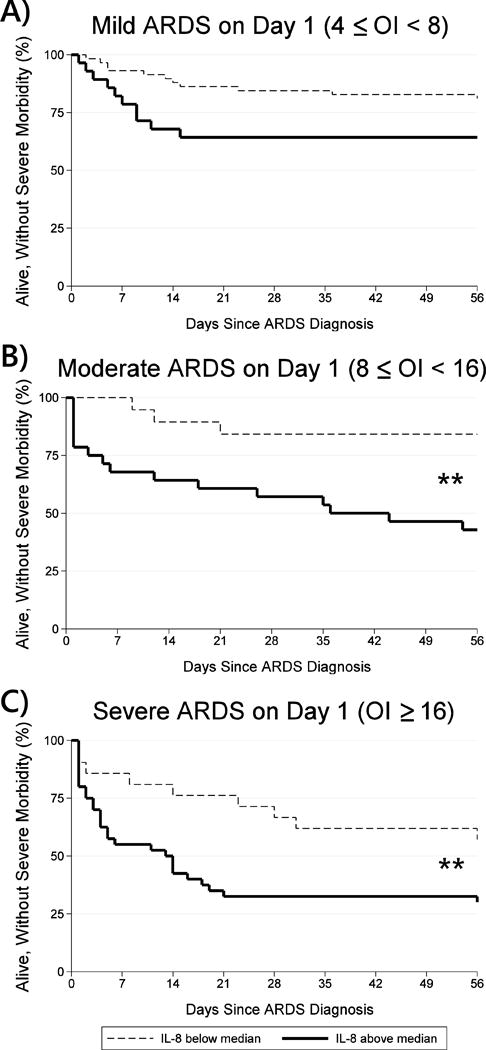
Legend: A) Among patients with mild ARDS (4≤OI<8, n=86), mortality or severe morbidity trended towards being more common amongst those with day 1 IL-8 above the median (36%, 10/28) than those below the median (19%, 11/58, p=0.090). Log-rank test of equality of Kaplan Meier functions p=0.061. B) Among patients with moderate ARDS (8≤OI<16, n=47), mortality or severe morbidity was more common amongst those with day 1 IL-8 above the median (57%, 16/28) than those below the median (16%, 3/19, p=0.005). Log-rank test of equality of Kaplan Meier functions p=0.006. C) Among patients with severe ARDS (OI≥16, n=61), mortality or severe morbidity was more common amongst those with day 1 IL-8 above the median (70%, 28/40) than those below the median (43%, 9/21, p=0.039). Log-rank test of equality of Kaplan Meier functions p=0.023.
DISCUSSION
In this study, we measured plasma levels of pro- and anti-inflammatory cytokines in 194 pediatric ARDS patients. We found a strong relationship between mortality and elevated plasma levels of both pro-inflammatory (IL-6, IL-8, IL-18, MIP-1β, TNF-α) and anti-inflammatory cytokines (IL-1RA, IL-10, and TNF-R2). These cytokines were also associated with ICU morbidity as measured by survivor PELOD score, early physiologic evidence of lung and multi-organ injury, as evidenced by P/F ratio, OI, and the PRISM-3 score, and biochemical evidence of endothelial injury, including elevated plasma Ang-2 and sTM. Finally, we found that the addition of inflammatory cytokines to the OI improves risk-stratification in a heterogeneous ARDS population.
In terms of significance, this is to our knowledge the largest multicenter study of inflammatory pathways in pediatric ARDS and the first of its size to identify significant early systemic cytokine perturbations in pediatric ARDS nonsurvivors. This important finding builds upon work done in both adult clinical studies and animal models, which have identified alveolar neutrophilic infiltration and cytokine release as hallmark findings of ARDS (10, 32–36). While several studies in pediatrics have associated cytokinemia with meconium aspiration, influenza, and sepsis, this is the first to study a broad range of pro- and anti-inflammatory cytokines in a rigorously defined ARDS framework (37–39).
In terms of prognostic information, we identified significantly improved risk-stratification when OI was combined with day 1 cytokines. For example, among patients with PALICC-defined severe ARDS, those with IL-8 levels above the cohort median had nearly twice the rate of mortality or severe morbidity than those with IL-8 levels below the cohort median (p=0.039). Also, when the top quartile of risk according to OI, IL-6, IL-8, IL-10, TNF-R2, and HCT was compared to the top quartile of risk according to OI alone, there was a 15% increase in relative risk for death or severe morbidity (McNemar’s p=0.025). If applied in a prospective fashion in our cohort, this expanded model would have identified an additional 5 children out of 47 in the top quartile days-to-weeks before the development of death or severe morbidity, allowing for enrollment in research protocols of experimental therapies.
Ultimately, improved risk-stratification is crucial for the identification of candidate high-risk subgroups for the development of new therapeutics, particularly in pediatrics where a major challenge of clinical trials has been attainment of goal accrual. Once they become widely available, rapid molecular assays could make bedside risk-stratification for clinical trials enrollment a feasible goal and could represent a paradigm shift for pediatric ICU research.
Our study is part of a growing body of pediatric critical care research to use a composite outcome combining mortality and morbidity (40, 41). Neither the mortality rate in our cohort (38/194) nor in that of Yehya et al (37/283) (42) is sufficiently high to power a multivariable logistic regression associating mortality with OI and multiple cytokines. Therefore, we generated a composite outcome of mortality or severe morbidity, defined as the top quartile of survivor PELOD (≥30). This outcome was met by 77/194 patients and facilitated an appropriately powered logistic regression allowing us to show that the composite biomarker of OI, IL-8, and TNF-R2 outperformed the model of OI alone (p=0.042). The composite outcome of mortality or severe morbidity is biologically appropriate, clinically relevant, and meets recent PALICC and CPCCRN recommendations for attention to morbidity as a significant PICU outcome (41).
In terms of subgroup phenotyping, we identified higher levels of IL-8, IL-18, IL-10, and TNF-R2 in HCT ARDS patients relative to than non-HCT ARDS patients. Recent data suggest that HCT patients may have unique inflammatory derangements after transplantation that might differentiate mortality risk (43, 44). Clinical experience with pulmonary dysfunction during neutrophil engraftment as well as early success of TNF-α inhibition support the exaggerated role of inflammation in this population (45, 46). Although our ability to draw further conclusions was limited by the size of this subgroup, this analysis is an important first step in identifying clinically-validated molecular markers that differentiate subgroups of risk. Further work in identifying unique molecular phenotypes is critical to understanding the pathophysiologic heterogeneity of pediatric ARDS.
Our study has several strengths. First, given the cohort size and diversity of patient characteristics, our results likely have external validity with respect to other academic PICUs. Second, in addition to assessing mortality, we assessed ICU morbidity and biochemical evidence of endothelial injury. The former has been recently emphasized by the PALICC and is particularly important given the lower mortality rates in children in comparison to adults (41, 47), whereas the latter lends biologic and mechanistic plausibility to our findings. Third, we increased our sample size by 15% by imputing PaO2 from SpO2 ≤97% in 26 patients lacking an arterial line but otherwise meeting enrollment criteria.
Our study has some limitations. First, our models were derived in a large pediatric cohort but do require external validation. We attempted to address this by using 5-fold internal cross-validation to minimize error without reducing sample size (30). Second, we did not mandate a uniform ventilator weaning protocol and could not account for variability in ventilator management patterns as they might influence our primary outcomes.
CONCLUSIONS
In conclusion, there was a significant association between mortality and elevated day 1 plasma cytokines in children with ARDS. These cytokines were also associated of ARDS illness severity, including the P/F ratio, OI, and PRISM-3 score; with ICU morbidity, including survivor ICU LOS and PELOD score; and with biochemical evidence of endothelial injury, including elevated plasma Ang-2 and sTM. These cytokines improve the prognostic performance of the OI in discriminating risk of mortality or severe morbidity and may allow for identification and enrollment of high-risk subgroups for future clinical trials.
Supplementary Material
Acknowledgments
We would like to thank our patients and their families for participating in this research. We also would like to thank the following collaborators for their assistance recruiting patients for this study: Heidi Flori, MD, Children’s Hospital Oakland; Robinder Khemani, MD, Children’s Hospital Los Angeles; Ana Graciano, MD, Children’s Hospital Central California; and Juan Boriosi, MD, American Family Children’s Hospital.
Funding: This work was supported by the NIH NICHD K12HD000850 (Zinter); the NIH NHLBI K23HL085526 and R01HL114484 (Sapru), R36HL51856 (Matthay), and R01HL131621 (Calfee); and the NIH NCATS UL1TR000124 (UCLA CTSI).
Financial Disclosures: Dr. Matthay reports personal fees from Roche Genentec (Chair DSMB), personal fees from GlaxoSmithKline (DSMB), grants from GlaxoSmithKline, personal fees from Cerus Therapeutics, personal fees from Biogen, personal fees from Quark Pharmaceuticals, and grants from Amgen, all outside the submitted work. Dr. Calfee reports grants and personal fees from GlaxoSmithKline and personal fees and non-financial support from Boehringer Ingelheim, all outside the submitted work.
Dr. Zinter received support for article research from the National Institutes of Health (NIH), and he disclosed grants from the NIH and from the Pediatric Blood and Marrow Transplant Foundation. Dr. Spicer received support for article research from the NIH. Dr. Alkhouli received funding from the National Heart, Lung, and Blood Institute (NHLBI), and received support for article research from the NIH. Dr. Calfee’s institution received funding from GlaxoSmithKline, and she received funding from GlaxoSmithKline, Boehringer Ingelheim, and Bayer (consulting), and received grants from the NIH. Dr. Matthay received a grant from NHLBI and NIAID, and his institution received funding from GlaxoSmithKline (sepsis grant) and Amgen (experimental acute lung injury grant); he received funding from Biogen (consulting ARDS), Quark Pharmaceuticals (consulting ARDS), Roche Genentec (Chair DSMB for trials), Cerus Therapeutics (consulting), Thesan Pharmaceuticals (consulting), Incardia (consulting), GlaxoSmithKline (consulting), Bayer (consulting), Boehringer Ingelheim (consulting), and Biomarck Pharmaceuticals (consulting) Dr. Sapru’s institution received funding from the NIH, and he received support for article research from the NIH.
Footnotes
Author Contributions:
Study concept and design: Zinter, Sapru. Acquisition of data: Zinter, Orwoll, Spicer, Alkhouli. Analysis and interpretation of data: Zinter, Orwoll, Calfee, Matthay, Sapru. Drafting of the manuscript: Zinter. Critical revision of the manuscript for important intellectual content: Zinter, Orwoll, Calfee, Matthay, Sapru. Statistical analysis: Zinter, Sapru. Administrative, technical, or material support: Zinter, Alkhouli, Sapru. Study supervision: Sapru. Approval of final manuscript: Zinter, Orwoll, Spicer, Alkhouli, Calfee, Matthay, Sapru.
Copyright form disclosure:Dr. Orwoll disclosed that he does not have any potential conflicts of interest.
References
- 1.Sapru A, Flori H, Quasney MW, et al. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16:S6–22. doi: 10.1097/PCC.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman JJ, Akhtar SR, Caldwell E, et al. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124:87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 5.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal A, Zhuo H, Brady S, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: Results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012;303:L634–9. doi: 10.1152/ajplung.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kangelaris KN, Prakash A, Liu KD, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1102–13. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juss J, Herre J, Begg M, et al. Genome-wide transcription profiling in neutrophils in acute respiratory distress syndrome. Lancet. 2015;385(Suppl 1) doi: 10.1016/S0140-6736(15)60370-1. S556736(15)60370-1. [DOI] [PubMed] [Google Scholar]
- 9.Dobyns EL, Eells PL, Griebel JL, et al. Elevated plasma endothelin-1 and cytokine levels in children with severe acute respiratory distress syndrome. J Pediatr. 1999;135:246–249. doi: 10.1016/s0022-3476(99)70029-6. [DOI] [PubMed] [Google Scholar]
- 10.Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–31. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 11.Maniatis NA, Kotanidou A, Catravas JD, et al. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008;49:119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 13.Zinter MS, Spicer A, Orwoll BO, et al. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol. 2015 doi: 10.1152/ajplung.00336.2015. ajplung.00336.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orwoll BE, Spicer AC, Zinter MS, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): A prospective observational cohort study. Crit Care. 2015;19:435. doi: 10.1186/s13054-015-1145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA-NIH Biomarker Working Group. BEST (biomarkers, EndpointS, and other tools) resource. 2016 [PubMed] [Google Scholar]
- 17.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 18.Fuzery AK, Levin J, Chan MM, et al. Translation of proteomic biomarkers into FDA approved cancer diagnostics: Issues and challenges. Clin Proteomics. 2013;10 doi: 10.1186/1559-0275-10-13. 13-0275-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, et al. The american-european consensus conference on ARDS. definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: Prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 21.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Brown SM, Grissom CK, Moss M, et al. Nonlinear imputation of Pao2/Fio2 from Spo2/Fio2 among patients with acute respiratory distress syndrome. Chest. 2016;150:307–313. doi: 10.1016/j.chest.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sack U, Burkhardt U, Borte M, et al. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998;5:28–32. doi: 10.1128/cdli.5.1.28-32.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann F, Albert MH, Arenz S, et al. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur Cytokine Netw. 2005;16:283–288. [PubMed] [Google Scholar]
- 25.Wiegering V, Eyrich M, Wunder C, et al. Age-related changes in intracellular cytokine expression in healthy children. Eur Cytokine Netw. 2009;20:75–80. doi: 10.1684/ecn.2009.0149. [DOI] [PubMed] [Google Scholar]
- 26.Zinter MS, Dvorak CC, Spicer A, et al. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. 2015;43:1986–1994. doi: 10.1097/CCM.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinter MS, DuBois SG, Spicer A, et al. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014;40:1536–1544. doi: 10.1007/s00134-014-3389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spicer AC, Calfee CS, Zinter MS, et al. A simple and robust bedside model for mortality risk in pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2016 doi: 10.1097/PCC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efron B, Hastie T, Johnstone I, et al. Least angle regression. The Annals of Statistics. 2004;32:407. [Google Scholar]
- 30.Vittinghoff E. Regression methods in biostatistics: Linear, logistic, survival, and repeated measures models. 2. New York: Springer; 2012. [Google Scholar]
- 31.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 32.Calfee CS, Ware LB, Glidden DV, et al. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med. 2011;39:711–717. doi: 10.1097/CCM.0b013e318207ec3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct versus indirect ARDS in single and multi-center studies. Chest. 2014;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovach MA, Stringer KA, Bunting R, et al. Microarray analysis identifies IL-1 receptor type 2 as a novel candidate biomarker in patients with acute respiratory distress syndrome. Respir Res. 2015;16 doi: 10.1186/s12931-015-0190-x. 29-015-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matute-Bello G, Downey G, Moore BB, et al. An official american thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krupa A, Fol M, Rahman M, et al. Silencing bruton’s tyrosine kinase in alveolar neutrophils protects mice from LPS/immune complex-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307:L435–48. doi: 10.1152/ajplung.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall MW, Geyer SM, Guo CY, et al. Innate immune function and mortality in critically ill children with influenza: A multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki K, Kondo M, Kato M, et al. Serum cytokine and chemokine profiles in neonates with meconium aspiration syndrome. Pediatrics. 2008;121:e748–53. doi: 10.1542/peds.2007-1697. [DOI] [PubMed] [Google Scholar]
- 39.Alder MN, Lindsell CJ, Wong HR. The pediatric sepsis biomarker risk model: Potential implications for sepsis therapy and biology. Expert Rev Anti Infect Ther. 2014;12:809–816. doi: 10.1586/14787210.2014.912131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 41.Pollack MM, Holubkov R, Funai T, et al. Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: A new paradigm for outcomes assessment. Crit Care Med. 2015;43:1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43:937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 43.Doring M, Cabanillas Stanchi KM, Mezger M, et al. Cytokine serum levels during post-transplant adverse events in 61 pediatric patients after hematopoietic stem cell transplantation. BMC Cancer. 2015;15 doi: 10.1186/s12885-015-1616-z. 607-015-1616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nomura S, Ishii K, Inami N, et al. Evaluation of angiopoietins and cell-derived microparticles after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:766–774. doi: 10.1016/j.bbmt.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Yanik GA, Grupp SA, Pulsipher MA, et al. TNF receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome (IPS). A joint pediatric blood and marrow transplant consortium (PBMTC) and children’s oncology group (COG) study (ASCT0521) Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khandelwal P, Mellor-Heineke S, Rahman N, et al. Cytokine profile of engraftment syndrome in pediatric hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Flori H, Dahmer MK, Sapru A, et al. Comorbidities and assessment of severity of pediatric acute respiratory distress syndrome: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:S41–50. doi: 10.1097/PCC.0000000000000430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


