Abstract
Objectives
Links between microbial alterations and systemic inflammation have been demonstrated in chronic disease, but little is known about these interactions during acute inflammation. This study investigates the effect of dietary supplementation with cellulose, a nonfermentable fiber, on the gut microbiota, inflammatory markers, and survival in two murine models of sepsis.
Design
Prospective experimental study.
Setting
University laboratory. Subjects: 6 week old male C57BL/6 wild-type mice.
Interventions
Mice were assigned to low-fiber (LF), normal-fiber (NF), or high-fiber (HF) diets with or without antibiotics for two weeks and then subjected to sepsis by cecal ligation and puncture (CLP) or endotoxin injection. Fecal samples were collected for microbiota analyses before and after dietary interventions.
Measurements and Main Results
Mice that received a HF diet demonstrated increased survival after CLP relative to mice receiving LF or NF diets. The survival benefit was associated with decreased serum concentration of pro-inflammatory cytokines, reduced neutrophil infiltration in the lungs, and diminished hepatic inflammation. The HF diet also increased survival after endotoxin injection. Bacterial 16S rRNA gene sequences from each sample were amplified, sequenced, and analyzed. Fiber supplementation yielded an increase in relative abundance of the genera Akkermansia and Lachnospiraceae, taxa commonly associated with metabolic health. Administration of antibiotics to mice on the HF diet negated the enrichment of Akkermansia species and the survival benefit after CLP.
Conclusion
Dietary supplementation with cellulose offers a microbe-mediated survival advantage in murine models of sepsis. Improved understanding of the link between diet, the microbiota, and systemic illness may yield new therapeutic strategies for patients with sepsis.
Keywords: sepsis, critical illness, fiber, enteral nutrition, microbiome, gut microbiota, Akkermansia, Antibiotics, cecal ligation and puncture, endotoxemia, cellulose
Introduction
The role of gut bacteria in modulating immune and inflammatory responses during sepsis has not been well studied. Important recent research has clearly shown that the microbiome typically plays a central role in the training and function of the host immune system (1). In times of health, the host-microbe relationship supports the appropriate response to pathogens and tolerance to commensals (1). However, disturbance of this relationship can clearly lead to significant local and systemic inflammation. The links between microbial disturbances and systemic inflammation have been well studied in the setting of chronic disease, e.g. obesity (2), inflammatory bowel disease (3), and HIV infection (4). In the setting of an acute insult such as sepsis, it is unclear whether the constituents of the gut microbiome can impact the inflammatory response and clinical outcomes.
Dietary modification is increasingly recognized as a relatively simple method of modifying systemic inflammation via changes in the gut microbiota (5, 6). In particular, dietary fiber possesses well documented anti-inflammatory properties that can be explained in part by fiber-induced effects on the microbiota (7–11). Fiber is typically divided into classes of fermentable and nonfermentable (resistant) fibers, and to date the anti-inflammatory potential of fermentable fibers have been best studied (10, 12). Elegant work in preclinical models has demonstrated how fermentable fiber supplementation can modify disease outcomes via microbiota-induced changes in production of specific anti-inflammatory metabolites (13, 14).
Although the mechanisms are not yet elucidated, recent evidence suggests that supplementation with cellulose, a nonfermentable fiber, also exerts protective anti-inflammatory effects that may be mediated by the microbiome (15, 16). We hypothesized that cellulose supplementation would improve animal survival in murine models of sepsis by modulating the microbiome and decreasing systemic inflammation. We report that short term cellulose supplementation prior to a septic insult leads to a reproducible enrichment in protective gut microbial species and a survival benefit, and that these results are abrogated in the presence of antibiotics.
Materials and Methods
Animal experiments
All animal experiments were performed with approval from the University of Pittsburgh IACUC, and care of the animals was in accord with NIH guidelines for animal treatment. Twenty four hours after arrival, C57BL/6 mice (6-8 weeks old) were randomized to receive chemically defined diets (DyetsInc, Bethlehem, PA) containing low (LF) fiber concentration (0.4%), normal (NF) fiber concentration (5%), or high (HF) fiber concentration (30%) (n=24 in each group). The content of diets (Table S1 in supplemental digital content) was otherwise comparable although the HF diet contained fewer calories due to increased cellulose content. Mice were not pair fed but daily weights were monitored routinely. After 2 weeks of the dietary intervention, sepsis was induced by cecal ligation and puncture (CLP) or endotoxin injection. In the CLP model, mice (n=24-38/group) were anesthetized with a 2% isoflurane/oxygen inhalational gas and CLP was performed under aseptic conditions. A 1 cm midline incision was made in the abdomen and the cecum was delivered into the wound, ligated 1 cm from the cecal tip using a single suture, and then punctured with a single pass of a 20-gauge hypodermic needle. The cecum was gently squeezed to extrude a small amount of feces from the perforation sites and returned to the peritoneal cavity. The laparotomy was closed with silk sutures. Sham controls were subjected to the same procedures but without ligation and puncture of the cecum. Tissue and blood samples were collected at 1, 4 and 7 days after CLP. Mice were weighed weekly. Fecal samples during the dietary intervention and cecal samples at sacrifice were collected for microbiome profiling.
In the endotoxemia model, mice (n=14/group) received an intraperitoneal injection of lipopolysaccharide (30 mg/kg Escherichia coli LPS, serotype 0127:B8; Sigma, St Louis, MO, USA). Fecal samples during the dietary intervention and cecal samples at sacrifice were collected for microbiome profiling. For experiments involving antibiotics (n=14 in each group), ampicillin (1 g/l; Sigma, St Louis, MO), neomycin (1 g/l; Sigma, St Louis, MO), metronidazole (1 g/l; Sigma, St Louis, MO), and vancomycin (0.5 g/l; Sigma, St Louis, MO) were each added to the drinking water. The amount of water intake was comparable in the different treatment groups.
Tissue myeloperoxidase assay
The myeloperoxidase (MPO) assay was performed as previously described, with minor modifications (17). Frozen lung tissue (50 mg) was homogenized in 1 ml of 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate (ph 7.0) and centrifuged at 4,000 rpm for 30 min at 4° C. An aliquot of the diluted supernatant was allowed to react with 16 mM tetra- methyl- benzidine and 80 mM sodium polyphosphates (NaPP) (ph 5.5) for 5 minutes. Next, 0.004% H2O2 was added to each well for 3 minutes and the reaction was stopped with 500 ul of 2 M acetic acid. The rate of change of absorbance was measured by spectrophotometry at 650 nm, and data were expressed as the quantity of enzyme degrading 1 μmol of peroxide/min at 37° C in units per 100 mg weight of tissue.
Hepatic Histology
Identical segments of liver and lung from each animal were rapidly harvested at sacrifice, fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections (5μm) were cut from the paraffin blocks and stained with hematoxylin and eosin. The tissue sections were examined under light microscopy by a senior independent pathologist (S.R.) blinded to experimental groups.
Multiplex cytokine assay
Serum was collected by centrifugation at 2000 g for 5 min at 4°C, aliquoted, and stored at -70°C until analysis. A multiplex cytokine assay (M60009RDPD Bio-Rad, Hercules, CA, USA) was performed in accordance with the manufacturer's instructions using the Luminex 200 system instrument (Luminex Corporation).
Sequencing and analysis of bacterial 16S rRNA genes
Microbial DNA was extracted using MO BIO PowerSoil DNA Isolation kits using the manufacturer's protocol with modifications. 16S rRNA amplicons were produced utilizing primers targeting the V4 region using 515F and 806R primers. Primers utilized either the Illumina adaptor, primer pad and linker (forward primer) or Illumina adaptor, Golay barcode, primer pad and linker (reverse primer) followed by a sequence targeting a conserved region of the bacterial 16S rRNA gene as described in Caporaso et al. (18). All PCR amplicons were purified, quantified, and pooled in equimolar ratios as described in (19), with the exception that 30 PCR cycles were done. Sequencing was performed on the Illumina MiSeq.
QIIME (v1.9) was used to demultiplex raw sequence reads (20). UPARSE (v8.0) was used to quality filter the reads, cluster reads at a 0.97 operational taxonomic unit (OTU) threshold, and remove chimeric sequences (using the “gold database”) (21). UCLUST was used to assign taxonomy to predicted OTUs, using the the green genes reference database (v13.8). Alpha and beta-diversity comparisons were performed using QIIME was and taxonomic summaries were generated with QIIME.
Comparison of sample composition and identification of statistically significant differences was performed with LEfSe using correction for independent comparisons (22). Genera predicted to be biomarkers by LEfSe were excluded if their relative abundance was less than 0.01 on average in the sample set under consideration.
Statistical Analysis
Statistics are expressed as mean ± standard error of the mean (SEM) in all figures and tables. Kaplan–Meier survival curves were calculated for each treatment group, and differences between survival curves were analyzed by log rank tests (Sigmaplot 13.0, Systat Software, San Jose, CA, USA). Two-way analysis of variance (ANOVA) (Prism, Graphpad) was used to evaluate the effect of treatment on each immune parameter at each time point. When an independent variable had a significant P value, a posthoc t test with Bonferroni correction for multiple comparisons was performed.
Results
Supplementation with cellulose reduces inflammation and improves survival after cecal ligation and puncture
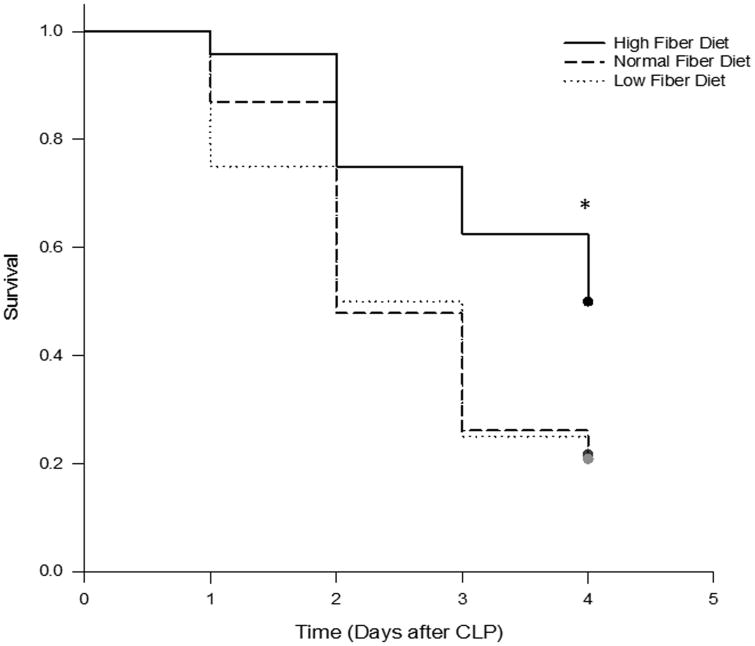
Mice were fed with either NF, LF or HF diets for two weeks and then subjected to CLP. During the two week feeding period, weight gain was similar across all three groups (10-13% above baseline). Among mice that were fed NF diet, the first deaths after CLP occurred at 24 h and overall mortality after CLP was 80% at 96 h (Figure 1). Mice fed with LF diet demonstrated a similar mortality rate of 70% at 96 h after CLP. However, mice that were fed HF diet demonstrated a delay in the onset of death and a significant reduction in overall mortality (50% at 96 h) (P=0.026).
Figure 1.
Cellulose supplementation increases survival after cecal ligation and puncture. Shown are Kaplan-Meier survival curves for mice fed LF, NF and HF diets for two weeks prior to CLP (n=24 in each group). Survival was significantly increased in HF diet mice relative to NF and LF diet mice (*, P =0.026; log-rank analysis).
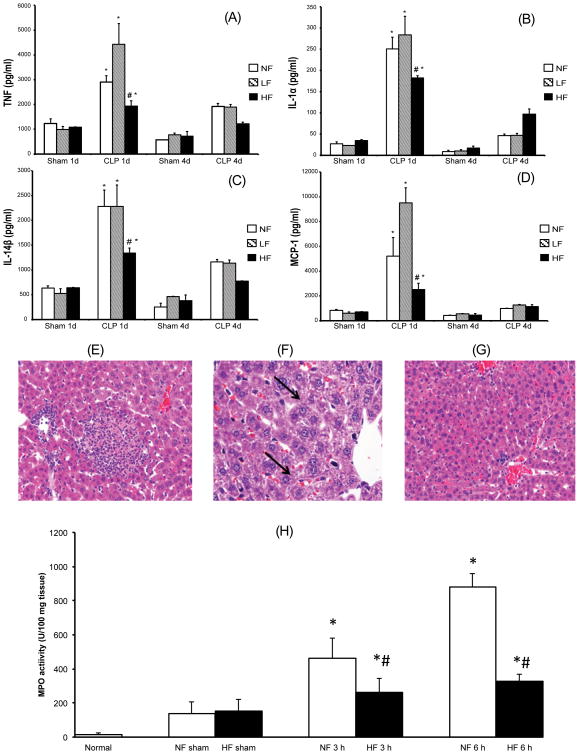
At one day after CLP, plasma concentrations of the pro-inflammatory cytokines tumor necrosis factor (TNF), interleukin (IL)-1ɑ and IL-1β and monocyte chemoattractant protein 1 (MCP-1) were significantly lower in mice fed HF diet compared to LF and NF dietmice (P<0.05) (Figure 2A-D). IL-1ɑ and IL-1β concentrations were similar in LF and NF diet groups, but interestingly TNF and MCP-1 were highest in the LF diet animals. In all three groups, plasma concentrations of the pro-inflammatory cytokines normalized four days after CLP, at which point there was no difference between the three groups (P>0.05) (Figure 2).
Figure 2.
Cellulose supplementation dampens the severity of systemic inflammation after cecal ligation and puncture. Shown are results for mice fed LF, NF and HF diets for two weeks prior to CLP or sham surgery. (A-D) Plasma concentrations at 1 day and 4 days after CLP are shown for TNFα (A), IL-1ɑ (B), IL-1β (C), and MCP-1 (D) are shown. Each data point represents the mean ± S.E.M. of 6 mice for each group. (*Represents P < 0.05 versus sham mice; # represents P < 0.05 versus mice fed with NF and LF diet and subjected to CLP) (E-G) Representative hepatic sections from NF, LF, and HF diet mice that were sacrificed 7 days after CLP. NF and LF diet mice demonstrate spotty hepatic necrosis, hemorrhage, neutrophilic infiltration and ballooning degeneration (E-F; bold arrows). In contrast, HF diet fed mice demonstrated a preservation of the hepatic architecture with infrequent necrosis, reduction in inflammatory cell infiltration and decreased ballooning degeneration (G). (H) MPO, an enzyme present in neutrophils, was measured as an index of neutrophil infiltration into the pulmonary tissue at 3 and 6 hours after CLP in the NF and HF diet mice. Each data point represents the mean ± S.E.M. of 6 mice for each group.(*Represents P < 0.05 versus sham mice; # represents P < 0.05 versus mice fed with NF and subjected to CLP).
At seven days after CLP in mice fed NF or LF diet, histologic examination of liver tissue sections revealed spotty hepatic necrosis, hemorrhage, neutrophilic infiltration, and ballooning degeneration (Figure 2E-F). Areas with portal vein congestion were also present. However, there was preservation of the hepatic architecture in HF diet mice with infrequent necrosis, reduction in inflammatory cell infiltration and decreased ballooning degeneration (Figure 2G). Preservation of pulmonary architecture was also seen at seven days (Supplemental Figure 1). Finally, we noted significantly lower MPO activity in the lung at 3 and 6 h after CLP in HF diet mice relative to NF diet mice (P<0.05) (Figure 2H). MPO activity in mice fed NF and LF diet was comparable (data not shown). Taken together, we have demonstrated that mice on the HF diet had increased survival after CLP associated with decreased serum pro-inflammatory cytokine expression at 24 hours, fewer areas of hepatic injury, and a reduction in neutrophilic infiltration in the lung.
Fiber supplementation promotes growth of protective microbial populations
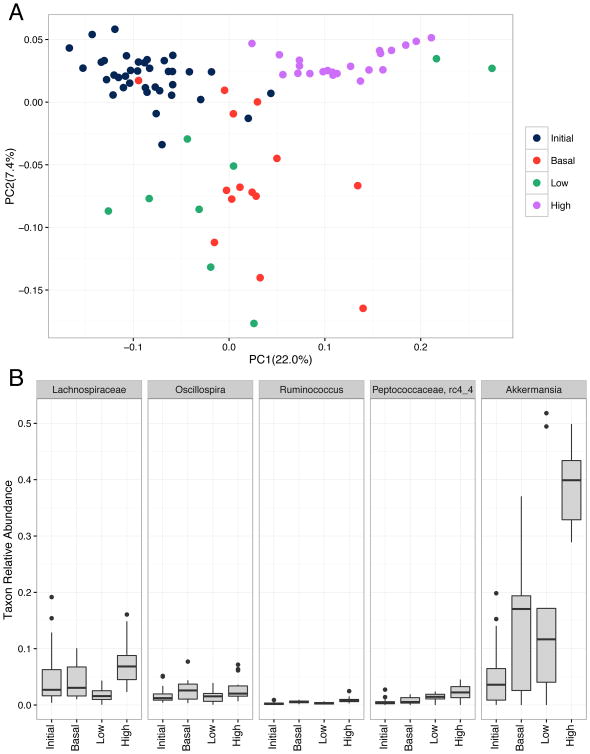
We employed bacterial 16S rRNA gene sequencing to profile the gut microbiota of animals in each group before and after the dietary interventions. We found no significant difference in alpha diversity (a metric of number of species present and their distribution) between initial samples and samples collected after two weeks of either LF, NF, or HF diets (observed species metric, nonparametric Monte Carlo test, 999 permutations, p-values > 0.05). By contrast, we observed significant differences in community composition (beta diversity) of the samples in each treatment group after 2 weeks of dietary intervention. Principal Component Analysis of all samples (Figure 3A) clearly demonstrates that the initial samples and samples collected 2 weeks after introduction of the HF diet cluster separately within PCoA space. The NF and LF samples overlap significantly rather than forming discrete clusters.
Figure 3.
Microbial diversity before and after dietary fiber supplementation with cellulose. Fecal samples were collected from animals before or after 2 weeks on a low (LF), normal (NF), and high fiber (HF) diet. (A) Principal coordinate analysis plot of weighted UniFrac distances between samples from the four groups. (B) Relative abundance of microbial taxa predicted to be enriched or depleted following cellulose supplementation. LEfSe was used to identify biomarkers (p<0.05) for either the initial, LF, NF, or HF sample groups. Taxa displayed here are those with high relative abundance across sample groups (> 0.01).
Specific genera enriched or depleted within experimental groups (Figure 3B) were identified using LEfSe. Notably we found that samples collected after 2 weeks of a HF diet were highly enriched with Akkermansia, a genus associated with metabolic health. They were similarly enriched with taxa from the family Lachnospiraceae, which contains beneficial anaerobes commonly found in the healthy colon. Interestingly, the NF and LF groups were each enriched with the genera Bifidobacterium, Allobaculum, and Enterococcus.
Antibiotic administration negates the protective effect of cellulose supplementation
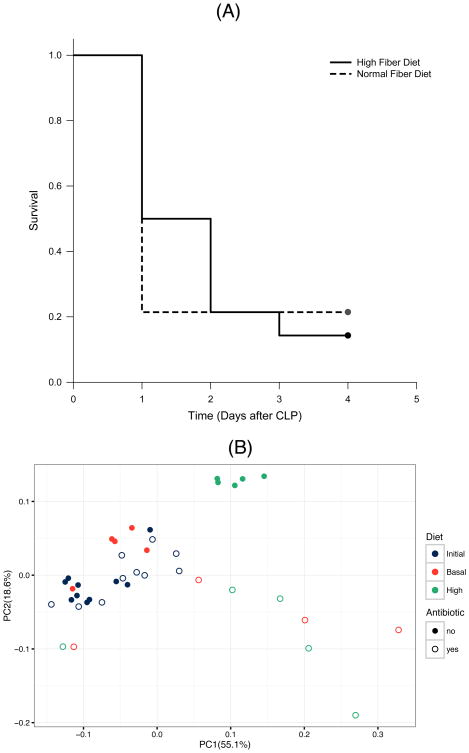
To explore whether the protective effect of the high cellulose diet is mediated in part by the gut microbiota, we exposed animals in the HF and NF diet groups to broad spectrum oral antibioticsin their drinking water for 2 weeks prior to CLP. We found that the survival benefit conferred by the HF diet was completely lost in the presence of antibiotics, indicating that an intact microbiome is required for the protective effect provided by cellulose supplementation (P<0.05) (Figure 4A). PCoA plot of unweighted UniFrac distances between samples demonstrates that fecal microbial populations of animals exposed to antibiotics no longer segregate according to cellulose supplementation (Figure 4B). The previously observed enrichment of Akkermansia after HF diet was not observed in the presence of antibiotic administration (mean relative abundances HF v NF, 0.022 v. 0.001, respectively).
Figure 4.
Antibiotic treatment reverses the benefits of cellulose supplementation. (A) Kaplan-Meier survival curves for mice fed NF and HF diets in the presence of antibiotics and subjected to CLP (n=14 in each group). HF diet fed mice had a significant (*, P < 0.05; log-rank analysis) difference in survival compared with NF diet fed mice, but this protective effect was completely lost in the presence of antibiotics (*, P > 0.05; log-rank analysis). (B) Changes in cellulose-induced microbiota changes in the presence of antibiotic therapy. Fecal samples were collected from animals before or after 2 weeks on a NF or HF diet, with or without treatment with broad-spectrum antibiotics. The principal coordinate analysis plot of weighted UniFrac distances between samples demonstrates that the tight clustering of HF samples is no longer observed in the presence of antibiotics.
Fiber supplementation improves survival in endotoxemia sepsis model
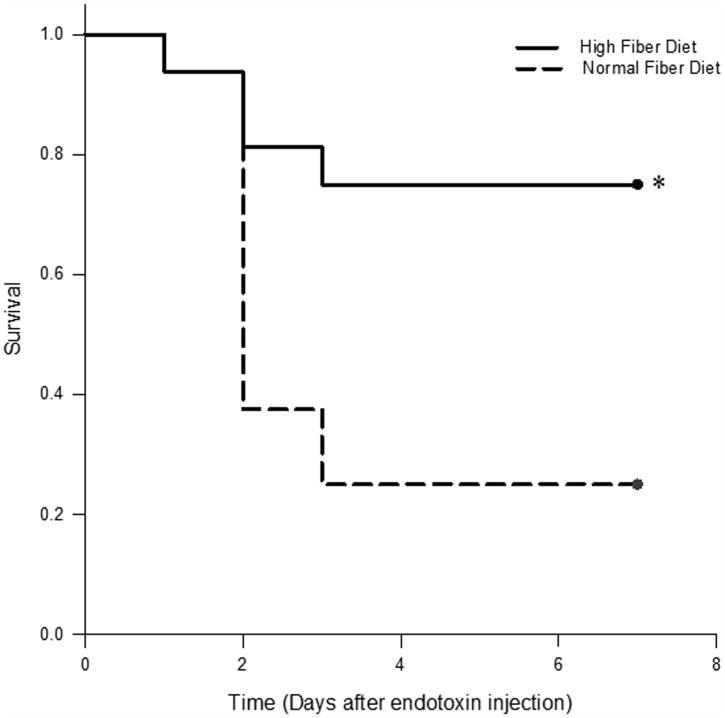
Finally, we sought to determine if the protective effect of a HF diet in a CLP model could be recapitulated in another model of sepsis, the murine endotoxemia model. To this end, mice fed with NF or HF diet for 2 weeks were administered intraperitoneal LPS (600 μg). Similar to the CLP cohort, approximately 80% of mice that were fed normal chow died within four days of LPS administration. In contrast, only 20% of mice fed a HF diet died after LPS administration (P<0.05) (Figure 5).
Figure 5.
Cellulose supplementation increases survival in murine model of endotoxemia. Kaplan-Meier survival curves for mice fed NF and HF diets and injected with LPS for induction of sepsis. HF diet fed mice had a significant (*, P < 0.05; log-rank analysis) difference in survival compared with NF diet fed mice.
Discussion
Septic shock is a common cause of death among critically ill patients (23). There is growing recognition that the morbidity from sepsis may derive both from an over exuberant pro-inflammatory response and/or an exaggerated anti-inflammatory response that creates a state of immunosuppression (24). Attempts to modulate outcomes and to identify predictive biomarkers after sepsis have generally considered the early pro-inflammatory state, but more recent studies have targeted anti-inflammatory mechanisms and the frequently observed post-sepsis state of immunosuppression. It has been suggested that establishing a heretofore undefined balance of pro- and anti-inflammatory mechanisms is a key to surviving sepsis. Host-specific variables such as age (25), gender (26), and preexisting comorbidities (27) have been shown to impact these processes.
It has been proven that the configuration of the gut microbiota can impact response to therapy in a range of clinical settings including diabetes (28) and cancer (29, 30). To date, however, clinical studies of sepsis to date have not accounted for the status of the gut microbiota, i.e. they have not accounted for which species are present in the gut at the time of disease. This is likely to change with the awareness that the commensal microbiota exerts previously unrecognized influences on immunity and inflammation. Indirect evidence that gut microbes impact survival after sepsis was provided by Johnson et al., who observed decreased risk-adjusted survival in patients receiving antibiotics in the 90 days prior to ICU admission for sepsis (31). In parallel, compelling work in animal models has demonstrated that the commensal microbiota can sharply impact the systemic response to bacterial infection and susceptibility to sepsis (32–35). Thus far, this work has focused upon innate immune responses, e.g. neutrophil homeostasis and production of reactive oxygen species, rather than the adaptive response. Taken together, these results suggest that specific organisms that are present in a specific patient at a particular point in time could impact the response of that patient to a septic insult.
Diet represents a powerful link between the gut microbiota and immune function (5, 6), and it appears that this relationship has relevance for survival in sepsis. Several groups have demonstrated that mice on a high fat diet experience increased mortality and organ dysfunction relative to controls in CLP and other murine models of sepsis (36–38). In humans, population-based studies indicate that a high-fat diet is associated with an increased lifetime risk of sepsis and increased biomarkers of inflammation (39, 40). These findings suggest that an individual's baseline characteristics, including the status of the microbiota, can impact the risk of experiencing adverse clinical outcomes.
In this study, animals receiving cellulose supplementation were partially protected against systemic inflammation and death in two murine models of sepsis. Previous work by Peck et al. demonstrated that calorie restriction improves survival in mice challenged with S. typhimurium (41). However, mice in that study lost 30% weight after three weeks of caloric restriction. In contrast, the average weight gain in mice in our study was 10-13% in all three groups. Specifically, the weight gain in the HF and NF diet mice was comparable (10.1 vs. 10.5%). Thus it is unlikely, that restriction of calories explains the significant reduction in overall mortality. Animals in this study also sustained clear changes in the gut microbiota, notably an enrichment for Akkermansia. This anaerobic organism is a commonly observed member of the human and rodent gut microbiome, and its abundance in humans has been inversely correlated with body weight (42) and inflammatory activity in IBD (43). Several reports have shown that expansion of A. muciniphilia populations is associated with improved glucose and adipose tissue metabolism, improved gut barrier function, and decreased systemic inflammation in mice and humans (44–47). These studies have also demonstrated that dietary supplementation with an oligofructose prebiotic reproducibly increases the abundance of A. muciniphilia, and that administration of A. muciniphilia to animals holds promise as a therapeutic strategy in diabetes, metabolic syndrome, and obesity.
Our data suggest that the protective effects of cellulose supplementation are microbiota-dependent, as the effects were abrogated in the presence of antibiotics. A simple potential explanation for this protection is that the organisms causing peritonitis in the CLP model are less virulent after fiber supplementation, but this would not explain the protective effect in the endotoxemia model which does not involve fecal spillage. Therefore, we favor the explanation that fiber supplementation alters the microbiota and host physiology such that it becomes possible to respond to injury with an appropriate inflammatory response compatible with survival. The details of this response must yet be clarified. It is possible that improved understanding of the link between diet, the microbiota, and systemic illness can yield new diet-based therapeutic strategies for patients suffering from sepsis.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Marco Fazzari and Dr. Francisco J. Schopfer for assistance with studies conducted for this manuscript.
Copyright Disclosure Form: Dr. Morowitz received support for article research from the National Institutes of Health (NIH). Dr. Caro institution received funding from NIH and disclosed work for hire. Dr. Ranganathan's institution received funding from NIH grants. Dr. Aneja's institution received funding from NIH. He received funding from Uptodate (royalty) and received support for article research from NIH.
Sources of support: NIH – R01 GM098474 (RKA)
Footnotes
The remaining authors disclosed that they do not have any potential conflicts of interest.
References cited
- 1.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 3.Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest. 2014;124:4190–4196. doi: 10.1172/JCI72330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the Gut Microbiota Is Associated with HIV Disease Progression and Tryptophan Catabolism. Sci Transl Med. 2013;5:193ra91–193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome, and immune system: envisioning the future. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015;148:1107–1119. doi: 10.1053/j.gastro.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Ajani UA, Ford ES, Mokdad AH. Dietary Fiber and C-Reactive Protein: Findings from National Health and Nutrition Examination Survey Data. J Nutr. 2004;134:1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 8.Chuang SC, Norat T, Murphy N, et al. Fiber intake and total and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr. 2012;96:164–174. doi: 10.3945/ajcn.111.028415. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy VMR, Wei G, Baird BC, et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300–306. doi: 10.1038/ki.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo SM. The Interplay Between Fiber and the Intestinal Microbiome in the Inflammatory Response. Adv Nutr Int Rev J. 2013;4:16–28. doi: 10.3945/an.112.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson HL, Campbell BJ. Review article: dietary fibre–microbiota interactions. Aliment Pharmacol Ther. 2015:n/a–n/a. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedlake L, Slack N, Andreyev HJN, et al. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis. 2014;20:576–586. doi: 10.1097/01.MIB.0000437984.92565.31. [DOI] [PubMed] [Google Scholar]
- 13.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 14.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 15.Cox LM, Cho I, Young SA, et al. The nonfermentable dietary fiber hydroxypropyl methylcellulose modulates intestinal microbiota. FASEB J. 2013;27:692–702. doi: 10.1096/fj.12-219477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy-Szakal D, Hollister EB, Luna RA, et al. Cellulose Supplementation Early in Life Ameliorates Colitis in Adult Mice. PLoS ONE. 2013;8:e56685. doi: 10.1371/journal.pone.0056685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods. 1996;198:1–14. doi: 10.1016/0022-1759(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 18.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costello EK, Lauber CL, Hamady M, et al. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 22.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Munford RS, Pugin J. Normal Responses to Injury Prevent Systemic Inflammation and Can Be Immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 25.Lowry SF. The stressed host response to infection: the disruptive signals and rhythms of systemic inflammation. Surg Clin North Am. 2009;89:311–vii. doi: 10.1016/j.suc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response--effect of gender differences. Injury. 2007;38:1382–1391. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pittet D, Thiévent B, Wenzel RP, et al. Importance of pre-existing co-morbidities for prognosis of septicemia in critically ill patients. Intensive Care Med. 1993;19:265–272. doi: 10.1007/BF01690546. [DOI] [PubMed] [Google Scholar]
- 28.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MT, Reichley R, Hoppe-Bauer J, et al. Impact of previous antibiotic therapy on outcome of Gram-negative severe sepsis*. Crit Care Med. 2011;39:1859–1865. doi: 10.1097/CCM.0b013e31821b85f4. [DOI] [PubMed] [Google Scholar]
- 32.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun. 2014;82:4596–4606. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deshmukh HS, Liu Y, Menkiti OR, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke TB. Microbial Programming of Systemic Innate Immunity and Resistance to Infection. PLoS Pathog. 2014;10:e1004506. doi: 10.1371/journal.ppat.1004506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strandberg L, Verdrengh M, Enge M, et al. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PloS One. 2009;4:e7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivera CA, Gaskin L, Singer G, et al. Western diet enhances hepatic inflammation in mice exposed to cecal ligation and puncture. BMC Physiol. 2010;10:20. doi: 10.1186/1472-6793-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan JM, Nowell M, Lahni P, et al. Short-term high fat feeding increases organ injury and mortality after polymicrobial sepsis. Obes Silver Spring Md. 2012;20:1995–2002. doi: 10.1038/oby.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutiérrez OM, Judd SE, Voeks JH, et al. Diet patterns and risk of sepsis in community-dwelling adults: a cohort study. BMC Infect Dis. 2015;15:231. doi: 10.1186/s12879-015-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peck MD, Babcock GF, Alexander JW. The role of protein and calorie restriction in outcome from Salmonella infection in mice. JPEN J Parenter Enteral Nutr. 1992;16:561–565. doi: 10.1177/0148607192016006561. [DOI] [PubMed] [Google Scholar]
- 42.Santacruz A, Collado MC, García-Valdés L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 43.Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 44.Everard A, Lazarevic V, Derrien M, et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. doi: 10.1073/pnas.1219451110. pnas.org. [DOI] [PMC free article] [PubMed]
- 46.Schneeberger M, Everard A, Gómez-Valadés AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.