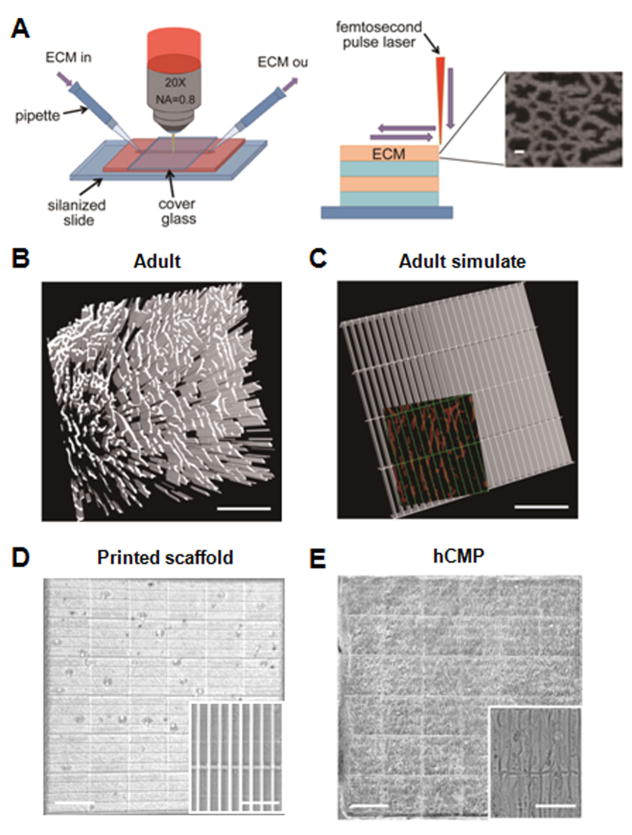
Figure 1. hCMP fabrication via 3D-MPE.
(A) The ECM and associated crosslinking solution are passed through the optical interrogation path while the laser power and dwell time are modulated to deposit ECM at each x, y location in each z plane. The submicron-scale features produced in the ECM scaffold are displayed in the inset (scale bar = 1 μm). Three-dimensional structures can be generated by combining multiple layers with the same or different ECM pattern. (B) Sections from the heart of an adult mouse were immunofluorescently stained for the presence of fibronectin and scanned via MPE (scale bar = 200 μm); then, (C) the distribution of fibronectin in the native tissue was simulated in a template. The simulated channels (green, 100 μm × 15 μm) are shown overlaying the fibronectin pattern of the native tissue (red) in the inset (scale bar = 100 μm). (D–E) The simulated template was used to determine the position of crosslinks in a solution of gelatin methacrylate, thereby producing a native-like ECM scaffold (D); then, the scaffold was seeded with hiPSC-derived CMs, ECs, and SMCs to generate the hCMPs (E). The complete hCMP is shown in the larger image (scale bar = 400 μm), while the individual channels and incorporated cells are visible in the inset (scale bar = 50 μm).