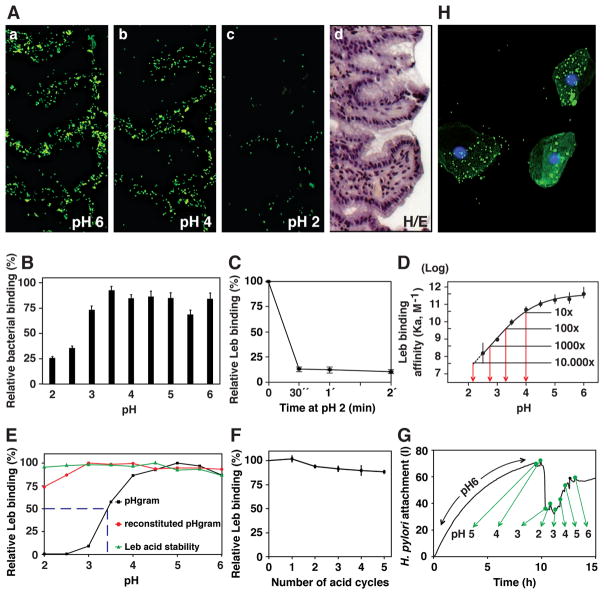
Figure 1. H. pylori Binding to Leb in Human Gastric Mucosa is Acid Sensitive, Responsive, Reversible, and Robust.
(A) In vitro binding of fluorescent H. pylori 17875/Leb (binds only to Leb) at (a) pH 6, (b) pH 4, and (c) pH 2 (quantified in Figure S1A); (d) H/E-stained adjacent section.
(B) H. pylori bacterial cells demonstrate pH dependence in binding to mucins (Lindén et al., 2004). Purified human gastric MUC5AC mucin was probed with gastric cancer (GC)-patient isolate (Y0 in Figure 6D) (means + SEM, n = 13 replicates).
(C) 17875/Leb bacteria were mixed with 125I-Leb-conjugate (125I-Leb) at pH 6 then acidified to pH 2. The proportion of Leb that remained bound was scored (means ± SD for n = 2).
(D) The 4 × 1011 M−1 affinity (Ka) at pH 6 showed log-fold reductions at pH 4, 3.3, 2.8, and 2.1 (arrows), and these are shown with 95% confidence intervals (CI).
(E) The acid sensitivity profile, here denoted pHgram (in black), was determined by a 1 h incubation with 125I-Leb at pH from 6 to 2. The dashed line shows when half of the Leb binding remained, i.e. 17875/Leb pH50 = 3.4. Reconditioning of bacterial cells to pH 5 after a 1 h exposure to acidic conditions and testing reactivation of Leb binding (in red) showed that the Leb glycan conjugate exhibited full acid stability at pH 2 (in green), which suggests that acid sensitivity involves the BabA protein itself.
(F) 17875/Leb bacteria were tested for Leb binding after each pH-cycle (means ± SD, n = 2 replicates).
(G and H) 17875/Leb bacterial binding in real time to recLeb-CHO cells (G and Figure S1G) or human BECs (H and Figure S1H) as measured by LigandTracer. The integrity of BECs (H) and recLeb-CHO cells (Figure S1G) was assessed at the end of the pH-cycle. Alexa488 was acid stabile (Figure S1E), and the restoration of Leb binding after neutralization resulted from pre-existing BabA protein, not de novo synthesis, because these experiments used previously frozen (dead) H. pylori.