Figure 2. Diversity in Acid Sensitivity in Binding to Leb Among Clinical Isolates is Encoded by the Protein Sequence of BabA.
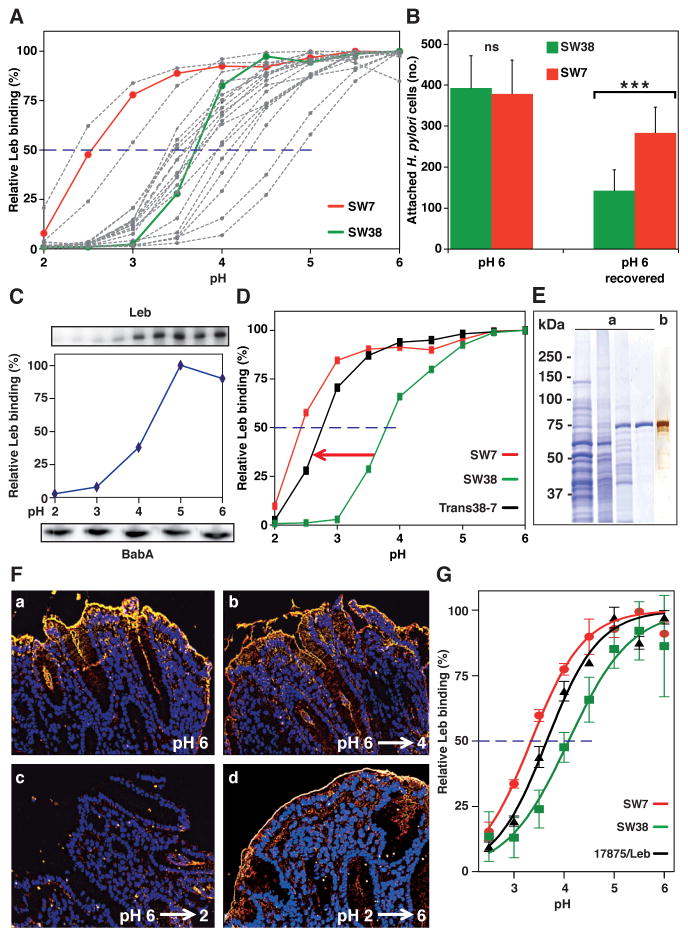
(A) Leb binding is displayed by pHgram in relative terms to facilitate inter-strain comparison in acid sensitivity in binding (absolute Leb binding is shown in Figure S2A). Each individual pHgram and pH50 value is highly reproducible (Figure S2B).
(B) Attachment at pH 6 and re-attachment after a full pH-cycle (Figure S2C, similar to Figure 1G) by strains SW7 (Alexa555, red) and SW38 (Alexa488, green) was significantly different as measured by Bonferroni post-hoc tests; means ± SD, n = 7 and n = 10 field views for SW7 and SW38, respectively.
(C) 17875/Leb whole-cell proteins were separated by SDS-PAGE, transferred to a membrane, and strips were probed with Leb (Ilver et al., 1998) at pH 2–6 (Leb-binding is shown at the top and quantified in the middle, and the confirmation of full BabA membrane retention is shown at the bottom).
(D) pHgrams of babA donor strain SW7, strain SW38, and the Trans38-7 transformant. SW7 and SW38 were chosen because of their divergence in pH50 (A) and sequence difference (44 amino acids (6%)) (Table S5; Figure S2F) but similar binding affinities (Aspholm-Hurtig at al., 2004). The Trans38-7’s ~25% reduction in gained acid resistance might come from its lower BabA expression, which was more similar to SW38 (Figure S2E).
(E) Protein purification of BabA from H. pylori bacterial cells. SDS-PAGE of (a) whole bacterial cell protein extract (lane 1), ZW-12 detergent bacterial extract (lane 2), proteins eluted from the CEX column (lane 3), and BabA eluted from the Leb column (lane 4) (Figure S2G). (b) Silver staining and MALDI–MS (Figure S2H) shows BabA purified to homogeneity with high yield (Table S1).
(F) Purified BabA was applied to gastric mucosa at pH 6 and then exposed to (a) pH 6, (b) pH 4, or (c) pH 2 followed by immunostaining (yellow) (quantified in Figure S2I); (d) BabA was acidified at pH 2, reconditioned at pH 6, then applied to gastric mucosa and immunostained.
(G) pHgrams of Leb-ELISA of BabA protein purified from H. pylori strains SW7, 17875/Leb and SW38 with pH50 values of 3.3, 3.7, and 4.1, respectively (means ± SD for n = 2).