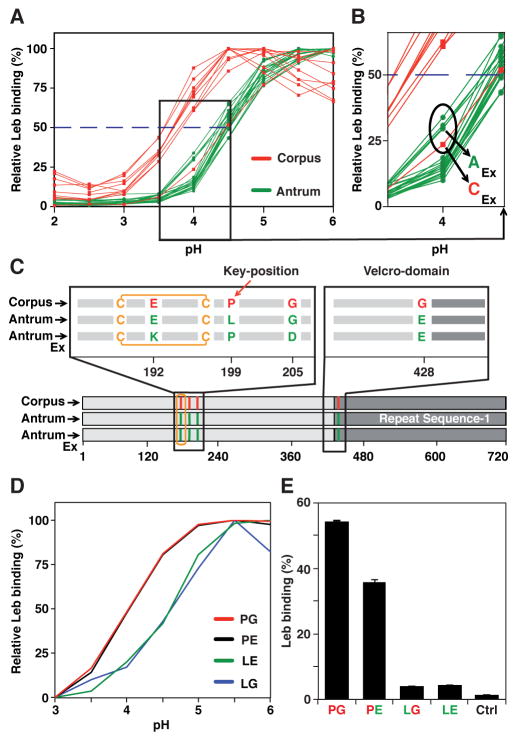
Figure 3. Gastric Antrum vs. Corpus Adaptation in Acid Sensitivity and Affinity in Binding is Encoded in BabA.
(A) pHgrams of 30 isolates from patient SO-2 from Orebro University Hospital, Sweden. The most and least acid sensitive isolates displayed a full pH unit difference.
(B) The Exceptional Antrum (AEx) and Corpus (CEx) isolates are circled.
(C) Location of the SO-2 BabA substitutions: P199L in Key-position and E192K/G205D in the Exceptional (Ex) isolates are all in the proximity of the fucose-binding CL2 disulfide-clasped loop (yellow) that constitutes the basis for the CBD. The G428E substitution is located diametrically opposite the CBD where it is part of Velcro Domain and is situated at the very junction of the Repeat-Sequence-1, i.e. the C-terminal 200 amino acid segment including the membrane anchored domain (Figure 4B) that is conserved between BabA, BabB and BabC (Alm et al., 2000).
(D) SPR-derived pHgram analyses of Leb-binding by E. coli recBabA526 protein from SO-2 corpus (P199/G428 (PG)), antrum (L199/E428 (LE)) and chimeric variants L199/G428 (LG) and P199/E428 (PE) (sensograms in Figure S3C).
(E) Leb binding by E. coli bacterial cells that express full-length recBabA720 from SO-2 corpus (PG), antrum (LE), and chimeric variants LG and PE was assessed by RIA. E. coli with no expression of recBabA was used as the control (Ctrl) (means + SD for n = 2).