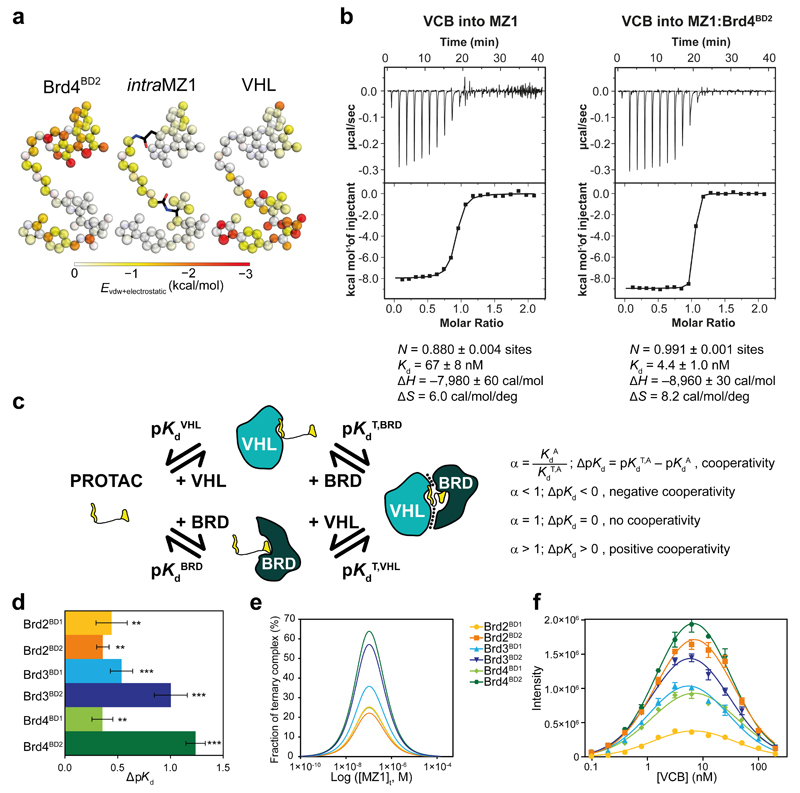
Figure 2. Brd4BD2 and VHL form a stable, cooperative complex in the presence of MZ1.
a, Novel ligand contacts are induced by ternary complex formation. Colour strength (from white to red) indicates the mean enthalpic energies of individual MZ1 atoms in contacting Brd4BD2 (left) or VHL (right), as well as intra-ligand contacts within MZ1 (centre) in a 100 ns MD simulation. b, Inverse ITC titrations of VCB into MZ1 (left, representative of eight replicates) and VCB into the pre-formed MZ1:Brd4BD2 (right, performed in duplicate) c, Ternary complex equilibria and definition of cooperativities. d, ΔpKd measured for VCB with MZ1 and the indicated BET-BDs, reported as difference (± uncertainty), from pKd values measured as mean (± 1 s.e.m.) as described in Online Methods. Statistical significance of pKd values for ternary titrations compared to the corresponding binary titrations was assessed by two-tailed t-test assuming equal variances, and is indicated as ** (p-value < 0.01) or *** (p-value < 0.001). e, Simulated fraction of ternary complexes based on mathematical model described in ref. 23. f, AlphaLISA intensity values titrating VCB against BET-BDs with MZ1. AlphaLISA intensities represent mean (± 1 s.d.) of intensity values from four technical replicates. The hook effect observed on these curves is due to biotinylated-VCB oversaturating the donor beads, resulting in a progressive decrease in signal.