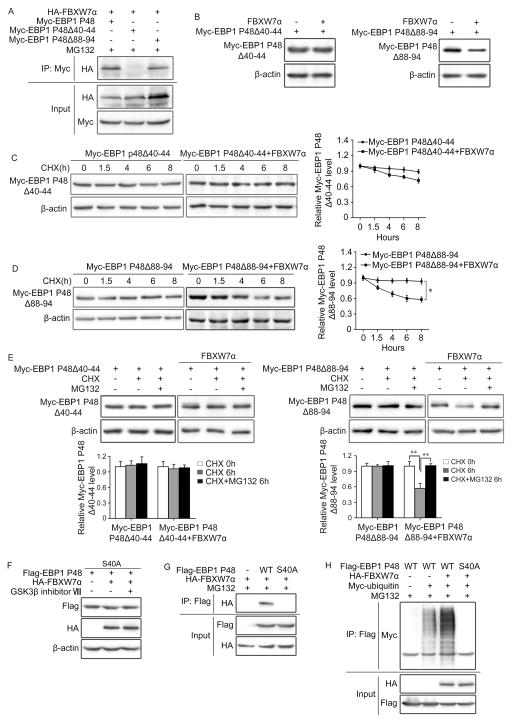
Figure 4. EBP1 P48 S40 is responsible for the binding to FBXW7.
A and B. HEK293T cells were co-transfected with HA-tagged FBXW7α and Myc-tagged EBP1 P48, Δ40–44 or Δ88–94 mutants. Immunoprecipitation followed by immunoblotting show that EBP1 P48 binds to FBXW7 through AA 40–44 (A) Western blotting show the degradation of EBP1 P48 by FBXW7 is AA 40–44 dependent (B). C and D. HEK293T cells were co-transfected with HA-tagged FBXW7α and Myc-tagged EBP1 P48Δ40–44 (C) or Myc-tagged EBP1 P48Δ88–94 (D), followed by treatment with 50μg/ml CHX for 0, 1.5, 4, 6 and 8 hours (h). Western blotting shows the degradation of EBP1 P48 mutants by FBXW7. E. HEK293T cells transfected as in (C, D) and treated with 50μg/ml CHX alone or plus 10μM MG132 for 6 hours (h). Western blotting shows the proteasomal degradation of EBP1 P48 is dependent on its AA 40–44. F. HEK293T cells were transfected with HA-tagged FBXW7α and Flag-tagged EBP1 P48S40A, Western blotting analysed whether EBP1 P48 S40A affected degradation by FBXW7 or sensitivity to the GSK3β inhibitor. G. HEK293T cells co-transfected with HA-tagged FBXW7α and Flag-tagged EBP1 P48 or P48 S40A. Immunoprecipitation followed by immunoblotting shows EBP1 P48 S40 is responsible for the binding to FBXW7. H. HEK293T cells co-transfected with HA-tagged FBXW7, Myc-tagged ubiquitin and Flag-tagged EBP1 P48 or EBP1 P48 S40A. Immunoprecipitation with Flag antibody followed by immunoblotting with Myc antibody confirmed that EBP1 P48S40 is responsible for the ubiquitination of EBP1 P48. All results are repeated at least three times. * P<0.05; ** P<0.01 based on Student t-test.