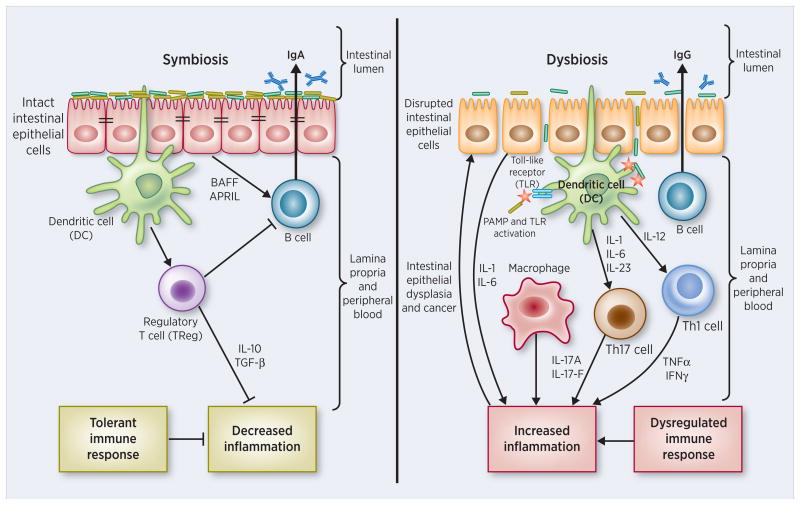
Figure 3. Dysbiosis: an immunocompromised state characterized by pathobiont colonization that leads to hyperinflammation, dysplasia and tumorigenesis.
(Left) Symbiosis: A symbiotic gut microbiota operates under a functional intestinal epithelial cell (IEC) barrier, with steady state proportions of mucus, pattern recognition receptors (PRRs), antimicrobial peptides, and secretory IgA, which in turn contain the microbiota in the intestinal lumen. Under tight control by IECs, the intestinal immune system within the gut lamina propria becomes largely tolerant to the resident commensals. Signaling cascades that occur downstream of toll-like receptors (TLRs) are used by IECs to detect microbes through PRRs. Upon lipopolysaccharide (LPS) stimulation of TLRs, the MYD88 protein is recruited, activating the NF-κB pathway, leading to production of antimicrobial proteins and proinflammatory cytokines. In a symbiotic gut, IECs are desensitized by repeated exposure to LPS or are attenuated by LPS-mediated downregulation of the IL-1 receptor–associated kinase 1 (IRAK1), an activator of the NF-κB cascade. Exposure to LPS induces epithelial cells to secrete TGF-β, B-cell-activating factor of the TNF family (BAFF), and a proliferation-inducing ligand (APRIL), all of which promote the development of tolerogenic responses to the microbiota. CD103+ dendritic cells (DCs) support the development of regulatory T (Treg) cells to secrete IL-10 and TGF-β, and together they stimulate the production of commensal-specific IgA. (Right) Dysbiosis: Increased intestinal exposure of diverse PAMPs, pro-inflammatory cytokines, apoptotic debris, and toxins leads to microbial dysbiosis and overgrowth of “pathobionts”, transformed symbiotic bacteria now under pathologic conditions. Pathobiont overgrowth leads to the loss of barrier integrity and a breach in the IEC barrier. Translocation of bacteria and bacterial components triggers the intestinal immune system through TLR activation, resulting in potentially harmful effector T cell responses set to clear invading bacteria. Ultimately, the secretion of IL-1 and IL-6 from IECs fuels a TH1 and TH17 response by DCs and macrophages and leads to higher levels of commensal-specific IgG by B cells.