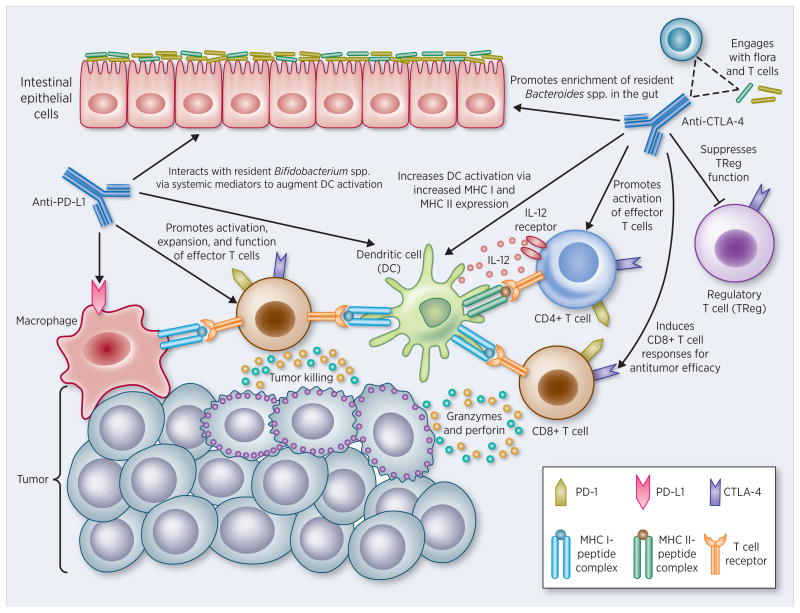
Figure 4. Gut microbiome directs the efficacy of immune checkpoint therapy.
Both anti-CTLA-4 and anti-PD-L1 therapies rely on gut microbiota for efficacy in immune activation. Anti-PD-L1 therapy has been shown to rely on the pre-existence of sufficient Bifidobacterium species, which are also thought to augment responses via PD-L1 binding on antigen-presenting cells such as DCs and macrophages. Subsequent ligation results in the prevention of suppressive signals to PD-1-expressing T cells. Similarly, anti-CTLA-4 indirectly alters the intestinal flora and enriches the Bacteroides species, possibly by promoting deterioration of the IEC barrier via activation of local lymphocytes. These bacteria then promote the activation of DCs, which present tumor antigens to prime and maintain anti-tumor T cell responses. Anti-CTLA-4 holds additional activation functions, including 1) preventing CTLA-4 from blocking activation of the co-stimulatory molecule CD28 on T cells and 2) blocking the immune-suppressive function of Tregs, which are required in deactivation of immune responses against tumors.