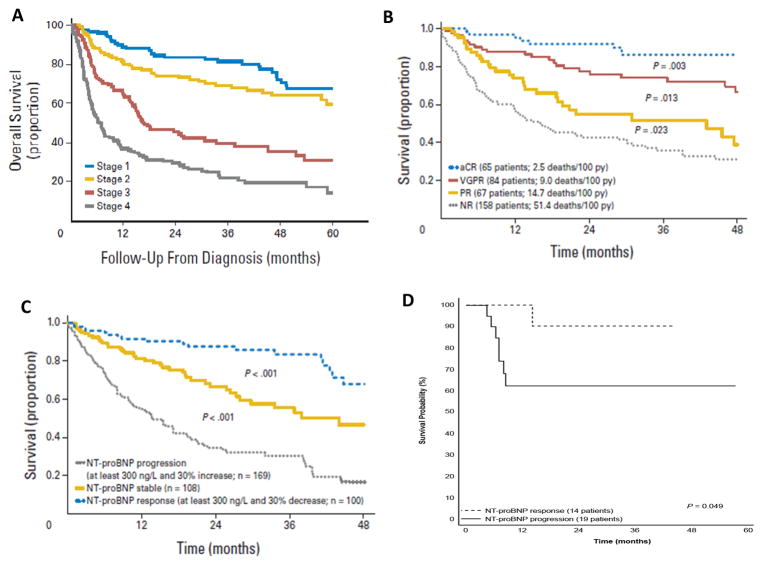
Figure 1.
Mayo staging system for risk stratifying subjects with AL-CA in which one point is assigned for each of the following: NT-pro-BNP ≥ 1800 pg/mL, Troponin T ≥0.025 ng/mL, and difference in serum free light chains ≥ 18 mg/dL. Those with highest score have the worst prognosis57 (Panel A).
Survival from the 3-month landmark of 300 patients with AL amyloidosis based on hematologic response. The proportion of stage III patients was not significantly different among the four hematologic response groups. CR, complete response; NR, no response; PR, partial response; VGPR, very good partial response and py, person-year; 60 (Panel B).
Prognostic relevance of cardiac response and progression criteria showing survival from the 6-month landmark of 377 patients with immunoglobulin light chain (AL) amyloidosis and baseline N-terminal natriuretic peptide type B (NT-proBNP)>650 ng/L according to NT-proBNP response and progression60 (Panel C).
Survival according to NT-proBNP response in an ongoing phase 3 trial comparing melphalan-dexamethasone with melphalan-bortezomib-dexamethasone (NCT01277016)61 (Panel D).