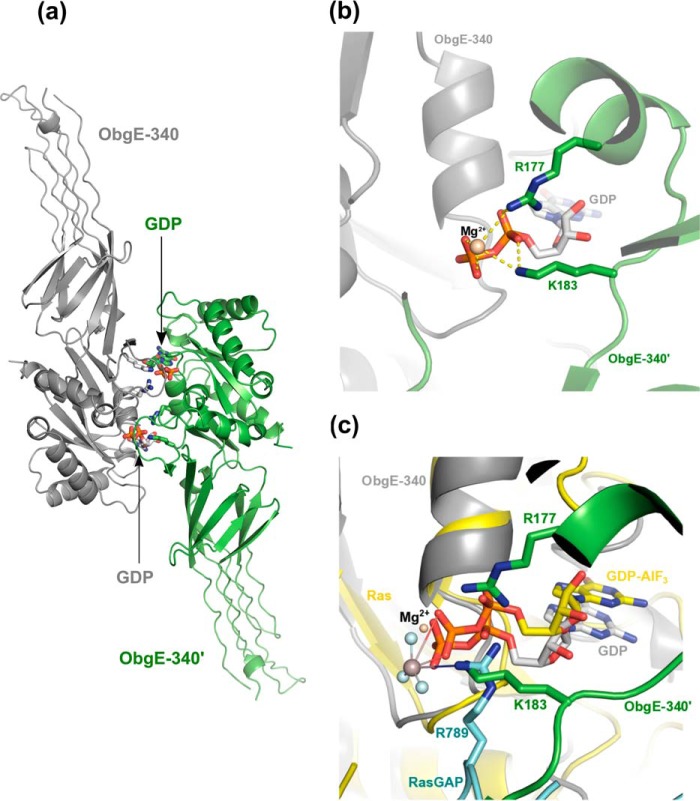
Figure 2.
Potential dimer interface of ObgE_340 generated though crystal symmetry operations. a, potential dimer organization of ObgE_340 via a face-to-face interaction of its G domain with the G domain of a neighboring protein molecule in the crystal lattice. The two symmetry variants are colored gray and green, and are labeled ObgE_340 and ObgE_340′, respectively. The C-atoms of the bound GDP molecules are colored according to the protomer to which they belong. b, close up view of the interaction surface shown in a. Lys-183 and Arg-177, located on the switch I and the first α-helix of the G domain of one protomer, interact with the phosphate groups of GDP from the adjacent promoter. c, superposition of the structure of the Ras-RasGAP complex (in presence of GDP-AlF3, PDB code 1WQ1 (62)) with the ObgE_340-GDP structure. The two symmetry related protomers of ObgE_340 and GDP are colored and labeled according to b. Ras and the bound GDP-AlF3 are colored yellow. The arginine finger of RasGAP (R789) is shown in cyan. This superposition shows a spatially similar position of the amino group of Lys-183 in the ObgE_340-ObgE_340′ homodimer and the guanido group of Arg-789 of RasGAP in the Ras-RasGAP complex.