Abstract
Inbred mice are commonly used to test candidate malaria vaccines, but have been unreliable for predicting efficacy in humans. To establish a more rigorous animal model, we acquired African woodland thicket rats of the genus Grammomys, the natural hosts for Plasmodium berghei. Thicket rats were acquired and identified as Grammomys surdaster by skull and teeth measurements and mitochondrial DNA genotyping. Herein, we demonstrate that thicket rats are highly susceptible to infection by P. berghei, and moderately susceptible to Plasmodium yoelii and Plasmodium chabaudi: 1–2 infected mosquito bites or 25–100 sporozoites administered by intravenous injection consistently resulted in patent parasitemia with P. berghei, and resulted in patent parasitemia with P. yoelii and P. chabaudi strains for at least 50% of animals. We then assessed efficacy of whole-organism vaccines to induce sterile immunity, and compared the thicket rat model to conventional mouse models. Using P. berghei ANKA radiation-attenuated sporozoites, and P. berghei ANKA and P. yoelii chemoprophylaxis vaccination approaches, we found that standard doses of vaccine sufficient to protect laboratory mice for a long duration against malaria challenge, are insufficient to protect thicket rats, which require higher doses of vaccine to achieve even short-term sterile immunity. Thicket rats may offer a more stringent and pertinent model for evaluating whole-organism vaccines.
Introduction
After decades of malaria vaccine development, a highly efficacious vaccine to prevent infection remains a top priority. Inbred mice have long been the animal model most commonly used to test candidate vaccines, yet have failed to predict outcomes in humans. For example, BALB/c mice develop sterile immunity against Plasmodium berghei (Pb) infection after a single immunization with 1,000 Pb ANKA radiation-attenuated sporozoites (RAS),1 and 90–100% protection against Plasmodium yoelii (Py) with very few PyRAS (750 given intravenously × 3 doses).2 In comparison, humans that received much higher doses and numbers of immunizations have achieved inconsistent levels of protection.3–6
Consideration of this difference led Chatterjee and others to investigate Pb in African woodland thicket rats (TRs) or Grammomys surdaster, a natural host–parasite combination. In this model, infection evolves in a chronic manner, similar to human infection and in contrast to infection in laboratory mice that frequently results in high parasite loads. Their studies suggested that TRs are highly susceptible to infection and are more difficult to protect via RAS immunization, comparable to what is observed in humans with Plasmodium falciparum.3,7 Herein, we seek to establish a thriving colony of TR, confirm previous findings, and test whether TRs are susceptible to other prominent strains of rodent malaria, including Py and Plasmodium chabaudi chabaudi (Pcc) strains. We also characterize whether RAS immunization or chemoprophylaxis vaccination (CVac) confer protection against various rodent malaria strains in TR. Finally, we confirm that TRs are a more stringent animal model than laboratory mice and therefore more pertinent to humans than are laboratory mice.
Materials and Methods
Rodent parasites.
Plasmodium berghei ANKA and Py 17XNL stabilates (cryopreserved, frozen, blood-stage parasites) were stored at the National Institutes of Health (NIH) Laboratory of Malaria Immunology and Vaccinology (LMIV), the Pb NK65 stabilate was obtained from the NIH Laboratory of Malaria and Vector Research (LMVR), and the Pcc AS and Pcc CB stabilates were obtained from Jean Langhorne, Division of Parasitology, Medical Research Council National Institute for Medical Research, London, United Kingdom.
TRs and mice.
African woodland TRs were originally captured in the wild in the south of the Democratic Republic of Congo, Africa, obtained from Department of Parasitology of Institut National de Recherche Biomédicale (INRB), and bred under pathogen-free conditions at NIH/National Institute of Allergy and Infectious Diseases (NIAID)/LMIV. TRs are outbred and housed as couples or harem for life. The offspring were weaned and removed from parents 21–28 days postdelivery and housed separately. Laboratory mice (C57BL/6, BALB/c, and CD1) were obtained from NIH-approved vendors. All breeding procedures and experiments were done in accordance with LMIV 1E ASP approved by the Institutional Animal Care and Use Committees.
Identification of TRs.
African woodland TRs were identified using four specimen heads and other body parts. DNA was extracted from biopsies taken from an external ear pinna from each, and 1,141 base pairs of mitochondrial DNA sequence were determined for each sample as previously described.8 Further identification was made by standard measurements of skulls and teeth, and by direct visual comparison with study skins and skulls in the National Museum of Natural History (NMNH).
Mosquitoes.
Three- to ten-day-old female Anopheles stephensi mosquitoes were obtained from the LMVR. The mosquitoes were infected by allowing them to feed on a donor mouse (BALB/c, CD1, or C57BL/6 mouse) during blood-stage parasitemia. The donor mouse selected for the feed exhibited gametocyte counts of 1+ or 2+ (one or two gametocytes per field for up to 10 fields) and a parasitemia level of less than 10%. The infected mosquitoes were reared at 24°C and 80% relative humidity for the Py strain, 26°C and 80% relative humidity for the Pcc strains, and 19–21°C and 80% relative humidity for the Pb strain. The infected mosquitoes were maintained on water containing 10% Karo syrup. Sporozoites (SPZ) were ready to use from 14 to 21 days post blood meal (dpb) for Py and Pcc strains, and from 18 to 25 dpb for the Pb strain.
Radiation and dissection of infected mosquitoes.
Infected mosquitoes were irradiated or attenuated with 10,000–15,000 rads (100–150 Gy) using a cesium irradiator. The irradiated or nonirradiated mosquitoes were placed in a freezer (−20°C) for 3–5 minutes to immobilize them. The immobilized mosquitoes were washed in a petri dish containing 70% ethanol for 1 minute and were dried using a Kim wipe (Kimberly-Clark Europe Limited, Reigate, Surrey, United Kingdom) and transferred to another petri dish containing 1× phosphate-buffered saline (PBS) or E199. Mosquitoes were dissected at room temperature and salivary glands were removed using a dissecting microscope (Zeiss Stemi 2000-C [Edmund Optics, Barrington, NJ] or Olympus SZ61 [Tokyo, Japan]), 3/10-mL insulin syringes (U10029G1/2), and forceps. One hundred pairs of dissected glands were put into a Protein LoBind 1.5-mL Eppendorf tube (Hamburg, Germany) containing 100 μL of dissecting medium, 5% normal mouse serum in PBS or E199 medium. After dissection, 0.2 mL of dissection medium was added to increase the total volume to 0.3 mL. A 1-mL syringe with a 26G needle attached was used to triturate and release the SPZ from the glands. The total volume of processed salivary gland was increased to 0.5 or 1.0 mL, depending on the expected SPZ yield per mosquito. SPZ were counted using a Phase contrast microscope (Nikon ECLIPSE E200 [Tokyo, Japan] or Zeiss Axiostar Plus [Gottingen, Germany]) and a disposable hemocytometer. Ten microliters of medium containing SPZ per sample were loaded in the hemocytometer and the SPZ concentration per milliliter was determined.
Infectious dose titrations in TR.
Using the nonirradiated SPZ concentration per milliliter, the highest infectious dose was calculated and serial dilutions (1:2) were made to titrate the amount of SPZ needed per TR (400, 200, 100, 50, and 25). TR, C57Bl/6, and BALB/c mice were infected with each dose titration using Pb ANKA SPZ, Pb NK65 SPZ, Py 17XNL SPZ, Pcc CB, and Pcc AS. Each infectious SPZ dose was administered in 200-μL dose volume intravenously via the tail vein. Daily blood smears were prepared on a slide and stained with 10% Giemsa to determine blood-stage parasitemia levels by microscopy.
Each TR was exposed to 5–8 infected mosquitoes at a time, and mosquitoes were replaced only once if they did not probe or feed. Each mosquito pint was fitted with a net screen through which the mosquitoes probed or bit for a blood meal. The test mosquitoes in the pints were immobilized in a −20°C freezer for 3 minutes and then examined under a dissecting microscope. The mosquitoes with blood meals were dissected, and only those with SPZ in their salivary glands were recorded as administering an infectious bite. The percent of infected mosquitoes used or the prevalence of infection of the mosquitoes used for the experiments ranged from 70% to 90%, depending on the batch.
RAS immunization and homologous challenge.
Using PbRAS, groups of 10 C57BL/6 mice and 10 TRs were immunized three times with 104 PbRAS at 3- to 4-week intervals, and another group of 10 TRs was immunized three times total with 105 PbRAS for the first dose, and 5 × 104 PbRAS for the second and third doses. Groups of five naïve C57Bl/6 mice and five TRs were used as controls. Four to five weeks after the last immunization, groups received an intravenous homologous challenge of 1,000 PbSPZ.
CVac and challenge.
Using the nonirradiated SPZ concentration per milliliter, the vaccination dose under chemoprophylaxis (pyrimethamine [PYR]) was calculated. PYR drug was administered orally via acidified drinking water from 5 hours before nonirradiated SPZ injection until up to 42 hours after nonirradiated SPZ injection. PYR drug water was then replaced with normal acidified drinking water. The following PYR drug stock concentration was prepared: 14 mg of PYR/mL of dimethyl sulfoxide with the stock diluted 100 times in acidified drinking water (1:100 = 3 mL of stock PYR: 297 mL of acidified drinking water). For intraperitoneal drug treatment, PYR stock concentration was diluted 100 times (1:100 = 100 of stock PYR: 9900 of PBS) and 200 μL of diluted PYR was administered intraperitoneally at the following time points: 20, 27, and 42 hours after nonirradiated SPZ injection or vaccination. After each vaccination, blood smears were collected on day 7 and 14 to check for blood-stage infections. Four to five weeks or 16 weeks after the last immunization, groups received an intravenous homologous challenge of 200 PySPZ.
Results
TRs are identified as G. surdaster.
To establish a more rigorous animal model for testing malaria vaccines, we acquired African woodland TRs of the genus Grammomys (Figure 1 ), the natural hosts for Pb. TRs were transferred from a colony at INRB in Kinshasa to establish a breeding colony at NIAID in Rockville, MD. TRs used in this study were originally captured in the wild at Fungurume (200 km from Lubumbashi) and Lumata (50 km from Lubumbashi) in the Katanga Province, south of the Democratic Republic of Congo, and have been maintained in a breeding colony at INRB, before transfer and rearing for these studies at LMIV. The areas where TRs were originally captured correspond with the regions where Pb strains were first isolated from G. surdaster and Anopheles dureni.9,10
Figure 1.
Photos of thicket rat (TR; Grammomys surdaster). Color photos of African woodland TR originally captured in the wild in the south of Democratic Republic of Congo, Africa, obtained from Department of Parasitology, Institut National de Recherche Biomédicale, and bred under pathogen-free conditions at National Institutes of Health/National Institute of Allergy and Infectious Diseases/Laboratory of Malaria Immunology and Vaccinology.
Animals were identified as G. surdaster by mitochondrial DNA genotyping and by skull and teeth measurements. All mitochondrial sequences from four independent animals were identical. When blasted to NCBI nonredundant protein database (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp), the top (best) hit was to accession number AF141218.1, for cytochrome B from a Grammomys species from Tanzania with 93% identity. The second best hit was to accession number EU349747.1 for G. surdaster with 86% identity. Comparatively, the percent identity to laboratory mice and laboratory rats was 83%, with approximately 880 other NCBI protein sequences of cytochrome B being more similar to TR than laboratory mice or rats. The methods used to identify our TR are previously described.11 Further identification was made by standard measurements of skulls and teeth taken with digital calipers accurate to 0.02 mm and compared with those in Table 2 of Dieterlen and others12 and the measurements listed in Happold and others.13 Species determinations were made by direct visual comparison with study skins and skulls in the NMNH. The skulls best match Grammomys dolichurus among the species represented in the NMNH collection, which is synonymous with G. surdaster by current taxonomy. This identification is consistent with current understanding of species distributions and definitions within Grammomys.12,13
TRs are susceptible to infection by rodent malaria species.
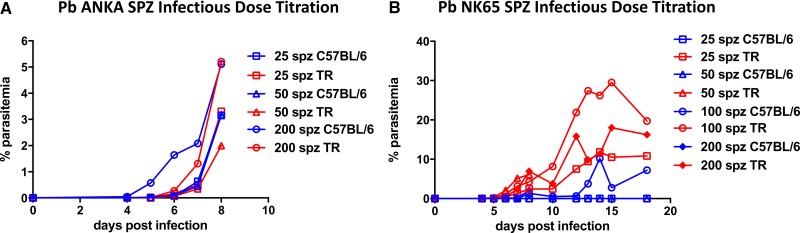
To establish reliable sporozoite doses to cause parasitemia, we first tested the susceptibility of TR to infection by parasites used in rodent malaria models. As Pb is commonly used in C57BL/6 mice, we dissected Pb ANKA and Pb NK65 salivary gland SPZ and compared their infectivity in TR to that in C57BL/6 mice. Initial infections determined that Pb ANKA was lethal to TR, and subsequent infection experiments required chloroquine cure once the blood-stage infection was imminent. Using dose titrations of between 25 and 200 SPZ injected intravenously, as few as 25 Pb ANKA (Figure 2A ) or Pb NK65 SPZ (Figure 2B) resulted in patency; mice and TRs appeared to have similar susceptibility to Pb ANKA, whereas susceptibility to Pb NK65 was as high or higher in TR than in mice. To assess infection by natural transmission, TRs were exposed to A. stephensi mosquitoes infected with Pb ANKA. Four of four TRs that provided blood meals to one or two sporozoite-infected mosquitoes became infected (Table 1). Two out of the three TR that did not provide blood meals to sporozoite-infected mosquitoes, nevertheless became infected presumably because they were probed by sporozoite-infected mosquitoes that did not take a blood meal. As few as one infected bite was sufficient to induce parasitemia.
Figure 2.
Thicket rats (TRs) are susceptible to infection with two strains of Plasmodium Berghei (Pb). (A) Dose-dependent parasitemia in three mice and three TRs inoculated with 25, 50, or 200 Pb ANKA sporozoites (SPZ). TRs were treated with chloroquine on day 6 after SPZ inoculation. (B) Parasitemia in four C57BL/6 mice and four TRs inoculated with 25, 50, 100, or 200 Pb NK65 SPZ. Each line represents one animal.
Table 1.
TR infection with Plasmodium berghei ANKA by mosquito bite
| TR | Fed mosquitoes | Fed mosquitoes with sporozoites | Parasitemia | |
|---|---|---|---|---|
| Experiment 1 | 1 | 5 | 1 | Positive |
| 2 | 3 | 2 | Positive | |
| 3 | 0 | 0* | Positive | |
| Experiment 2 | 1 | 3 | 1 | Positive |
| 2 | 2 | 2 | Positive | |
| 3 | 0 | 0* | Positive | |
| 4 | 1 | 0 | Negative |
TR = thicket rat.
Denotes mosquitoes that likely probed without taking a blood meal.
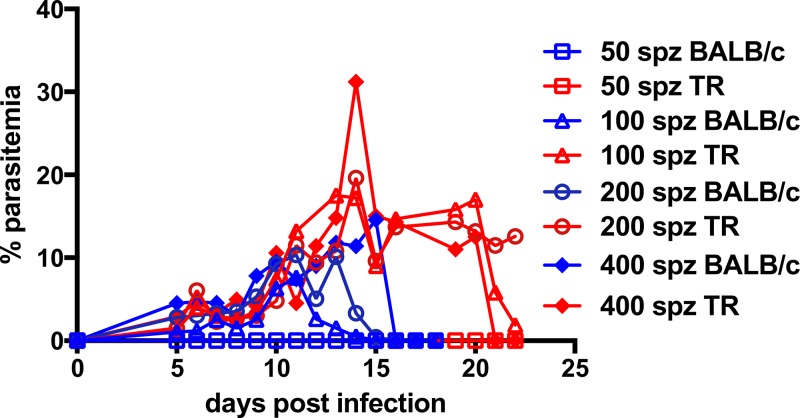
Next, Py 17XNL was used to infect TR and BALB/c mice. Salivary gland SPZ were dissected and injected intravenously in a dose range of 50–400 SPZ per animal. TRs were easily infected by Py, though not with the same sensitivity as seen with Pb, with 100 or more Py SPZ resulting in patency in both animal models (Figure 3 ). Interestingly, while mice and TR self-cured, TR appeared to require ∼1 week longer to clear parasitemia when SPZ were injected intravenously. We also confirmed infection by the natural route; two of four TRs that provided blood meals to sporozoite-infected mosquitoes became infected. As few as two mosquito bites were sufficient to result in patency (Table 2).
Figure 3.
Thicket rats (TRs) are susceptible to infection with Plasmodium yoelii (Py) 17XNL. Parasitemia in four BALB/c mice and four TRs inoculated with 50, 100, 200, or 400 Py 17XNL sporozoites (SPZ). Mice self-cured by day 16 postinfection, whereas TR also self-cured by day 23 postinfection. Each line represents one animal.
Table 2.
TR infection with Plasmodium yoelii 17XNL by mosquito bite
| TR | Fed mosquitoes | Fed mosquitoes with sporozoites | Parasitemia |
|---|---|---|---|
| 1 | 2 | 1 | Positive |
| 2 | 5 | 3 | Negative |
| 3 | 3 | 1 | Positive |
| 4 | 3 | 3 | Negative |
TR = thicket rat.
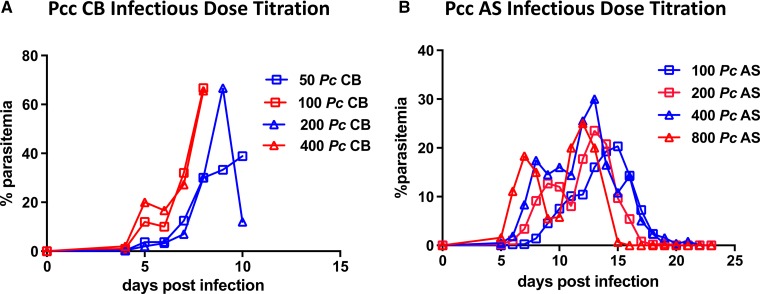
Finally, we confirmed TR were susceptible to Pcc CB (Figure 4A , Supplemental Figure 1) and AS (Figure 4B, Supplemental Figure 2) by both intravenous injection and mosquito bite (Table 3). Two of four TRs that provided blood meals to sporozoite-infected mosquitoes became infected, and as few as three infectious mosquito bites were sufficient to result in patency. In each mosquito bite experiment, all mosquitoes that probed took a blood meal except for one feeding study in which Pb ANKA-infected mosquitoes probed without taking a blood meal; in this study, TRs were not fully anesthetized and thus not completely still during mosquito exposure, thus preventing the mosquitoes from taking a blood meal.
Figure 4.
Thicket rats (TRs) are susceptible to infection with Plasmodium chabaudi CB and AS. (A) Parasitemia in four TRs inoculated with 50, 100, 200, or 400 Plasmodium chabaudi chabaudi (Pcc) CB sporozoites (SPZ). (B) Parasitemia in four TRs inoculated with 100, 200, 400, or 800 Pcc AS SPZ. Each line represents one animal.
Table 3.
TR infection with Plasmodium chabaudi chabaudi CB by mosquito bite
| TR | Fed mosquitoes | Fed mosquitoes with sporozoites | Parasitemia |
|---|---|---|---|
| 1 | 3 | 2 | Negative |
| 2 | 3 | 2 | Positive |
| 3 | 6 | 2 | Negative |
| 4 | 5 | 3 | Positive |
TR = thicket rat.
Whole-sporozoite vaccines have greater efficacy in laboratory mice than TRs.
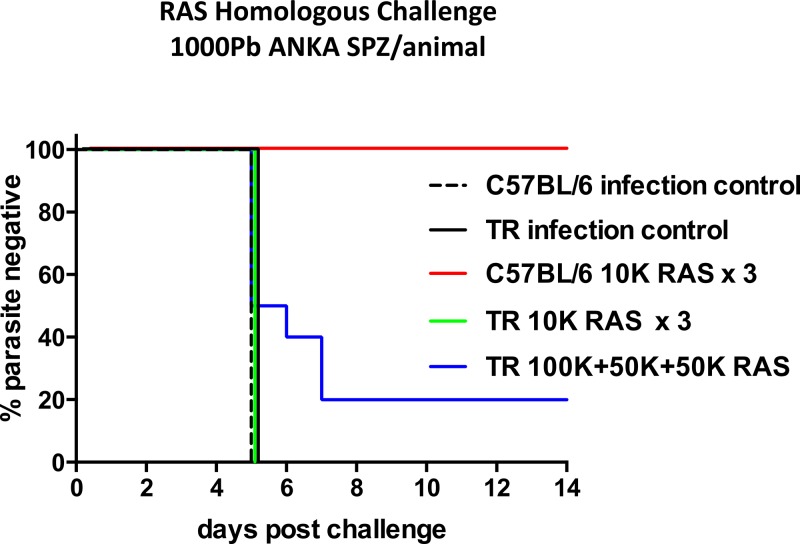
We assessed the potential of TRs as a model to study whole-organism vaccines. C57BL/6 mice were 100% protected (10/10) against challenge with 1,000 PbSPZ (Figure 5 ) after immunization with a total of 3 × 104 PbRAS administered in three doses (104 PbRAS per dose) at 3- to 4-week intervals. In contrast, this regimen protected 0% of TRs against challenge with 1,000 PbSPZ. A higher immunizing dose of 2 × 105 PbRAS administered in three doses at 3- to 4-week intervals (105 + 5 × 104 + 5 × 104) protected 20% of TRs from challenge with 1,000 PbSPZ (Figure 5).
Figure 5.
Radiation-attenuated sporozoites (RAS) immunization fully protects C57BL/6 mice, but elicits partial protection in thicket rat (TR) against high-dose homologous Plasmodium berghei (Pb) challenge. Percent parasite negative in C57BL/6 mice (N = 10) and TR (N = 10) immunized three times with 104 RAS 3–4 weeks apart, TR (N = 10) immunized three times with 105, 5 × 104, and 5 × 104 RAS 3–4 weeks apart, and naïve controls (N = 5 per group) challenged with 1,000 Pb ANKA SPZ.
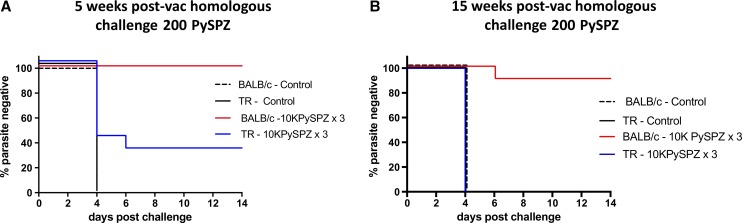
We next tested whole-sporozoite immunization of TRs against Py using nonattenuated SPZ administered under drug cover. Because we wanted to limit parasite development to the liver, we used PYR, which has been shown to be a successful approach with Pb in C57BL/6 and BALB/c mice.14 Using a CVac approach, we again observed differences between laboratory mice and TRs. After three doses of 3,000 Py 17XNL SPZ (PySPZ) under PYR drug cover at 3- to 4-week intervals, 75% of BALB/c mice versus 0% TRs were protected from challenge with 200 PySPZ (Table 4). After three doses of 104 PySPZ under PYR treatment at 3- to 4-week intervals, 100% of mice were protected but only 30% of TRs were protected from challenge with 200 PySPZ 5 weeks postvaccination (Figure 6A ). When challenge with 200 PySPZ was delivered 15 weeks postvaccination, 90% of the mice were protected and none of the TRs were protected (Figure 6B).
Table 4.
Plasmodium yoelii 17XNL pyrimethamine chemoprophylaxis vaccination against 200 P. yoelii SPZ challenge
| 3 × 103 SPZ × 3 | ||
|---|---|---|
| No. protected/no. challenged | % Protected | |
| TR | 0/4 | 0 |
| BALB/c | 3/4 | 75 |
TR = thicket rat; SPZ = sporozoite.
Figure 6.
High-dose chemoprophylaxis vaccination fully protects BALB/c mice but only partially protects thicket rat (TR) against homologous Plasmodium yoelii (Py) challenge. (A) Percent parasite negative in BALB/c and TR immunized three times via 104 Py sporozoites (SPZ) inoculation coupled with pyrimethamine (PYR) treatment and controls against challenge with 200 PySPZ 5 weeks postimmunization. (B) Percent parasite negative in BALB/c and TR immunized three times via 104 PySPZ inoculation coupled with PYR treatment and controls against challenge with 200 PySPZ 15 weeks postimmunization.
Discussion
Laboratory mice infected with rodent Plasmodium species such as P. berghei, P. yoelii, and P. chabaudi have been commonly used as proxies to investigate malaria in vivo. These models have been essential in developing an understanding of the mechanisms of infection in a mammalian host, the progression of the disease, host immune responses, and parasite genetics.15 Rodent infection models have also been instrumental in drug and vaccine development. However, many study results have been difficult to translate into clinical applications in humans, owing in part to the physiological and genetic differences of the host and parasite species used. In particular, vaccine studies in current mouse models of malaria have not been reliably predictive of human efficacy; laboratory mice are generally much easier to protect than humans. Although these models have been good tools to screen out ineffective vaccines, they have not been as successful in selecting effective vaccines.16 A more rigorous rodent model that is not easily protected via immunization would be useful to qualify or prioritize potential vaccine candidates.
A new phylogenetic analysis from the Sackler Institute for Comparative Genomics at the American Museum of Natural History shows that malarial parasites found in tree-dwelling rats share a sister relationship with P. falciparum and Plasmodium reichenowi.17 Although Plasmodium parasites in rodents differ from Plasmodium parasites in humans (e.g., the existence of the PfEMP1 antigen family in P. falciparum but not in rodent parasites18,19), individual gene analysis, regardless of methods, consistently shows that P. falciparum and P. reichenowi are sister taxa and the three species that infect rodents were always recovered as a monophyletic group.20,21 Many of the analyses placed P. falciparum and P. reichenowi as sisters to P. chabaudi, P. berghei, and P. yoelii as described previously by Perkins.17 Since the malarial parasites found in rodents share a sister relationship with P. falciparum, developing a TR model to study malaria may specifically accelerate progress toward the goal of a human malaria vaccine against P. falciparum.
Early studies by Yoeli in the 1960s and 1970s investigated the use of “tree rats” (Thamnomys surdaster) as experimental models for Pb infection, characterizing the course of infection in wild rats as chronically mild with low parasitemia, in contrast to the severe, fulminant disease observed in laboratory-bred species.22,23 Yoeli's studies also provided early insight to the histologic pathology in liver tissue induced by Pb sporozoite inoculation in T. surdaster.24 Subsequent to those early studies, rodents formerly known as T. surdaster are now referred to as G. surdaster.25
The TRs transferred from Kinshasa for these studies were identified by a team of experts from the Laboratory of Zoonotic Pathogens, Rocky Mountain Laboratories, and the NMNH Smithsonian Institute to be G. dolichurus, which is currently regarded as a synonym for G. surdaster. The name likely represents a species composite in need of taxonomic revision. Such a qualifier would simply acknowledge the appreciable increase in recognized rodent species that is occurring concurrent with the increased application of molecular tools to taxonomic problems.26 In the future, TRs in the northern part of the Democratic Republic of Congo may be separated out and referred to as G. surdaster, whereas the TRs in the south of Democratic Republic of Congo (LMIV TR) might continue to be referred to as G. dolichurus. The area where these TRs were captured is the same area where Pb was originally isolated,9,10 hence the evidence favors that this is the natural host of Pb.
Our results support the findings of Chatterjee and others,7 which suggested that TR (G. surdaster), the natural host for Pb, would be a more stringent rodent model to extrapolate to human malaria. TRs are highly susceptible to infection with doses as low as 25 Pb SPZ, and develop parasitemia after as few as one infected mosquito bite. We expand on previous findings by establishing TR susceptibility to other rodent forms of malaria including Py and Pcc, which resulted in patent parasitemia in at least 50% of animals.
Confirming previous findings, the natural host was more difficult to protect by RAS immunization in contrast to artificial hosts like inbred mice.7 Similar to humans who require immunization with > 1,000 mosquitoes carrying PfRAS to achieve sterile immunity,27 TRs required higher doses of RAS to achieve only partial protection against homologous challenge. We expanded on previous findings by testing CVac methods of immunization, and observed similar trends: higher doses of CVac using PYR were required for protection in some TRs versus all inbred mice. Similar SPZ doses were used in both the RAS and CVac studies, whereas RAS experiments were conducted using Pb and CVac studies were conducted using Py. Our results confirm that TRs are a more stringent rodent model for evaluating whole-organism vaccines for malaria and, as seen in humans, vaccine efficacy can be enhanced by increasing vaccine dose.28
The different requirements to achieve protection in TRs and artificial models may be due to different mechanisms of protection; for example, innate immunity characterized by the infiltration of mononuclear cells, neutrophils, and eosinophils is prevalent in artificial hosts, whereas it is absent in TRs.3,7 In addition, there may be differences in antigenic targets between the two models; for example, in laboratory mice, both immunizing and challenge SPZ were halted at the uninucleate, trophozoite stage, whereas development to submature schizonts was observed in Pb in TRs.3,7 Perhaps “the ‘adaptation’ of a parasite to an artificial host actually translates in immunological terms into defense mechanisms that in most circumstances are more effective than those seen in the natural host.”3
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Andre Laughinghouse and Kevin Lee of Laboratory of Malaria and Vector Research for providing all of the mosquitoes used in this project; Jean Langhorne for providing the Plasmodium chabaudi chabaudi parasites; Robert Morrison for helping with the thicket rat (TR) sequence analysis; Tom G. Schwan from the Laboratory of Zoonotic Pathogens in Montana, and Darrin Lunde and Michael Carleton from the Smithsonian for their work to identify TR; and J. Patrick Gorres for help with writing and submitting the manuscript.
Footnotes
Financial support: This research was supported and funded by the Intramural Research Program of the National Institute for Allergy and Infectious Disease of the National Institutes of Health.
Authors' addresses: Solomon Conteh, Charles Anderson, Lynn Lambert, Sachy Orr-Gonzalez, Jessica Herrod, Yvette L. Robbins, Dariyen Carter, and Patrick E. Duffy, Laboratory of Malaria Immunology and Vaccinology (LMIV), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Rockville, MD, E-mails: solomon.conteh@nih.gov, charles.anderson@nih.gov, lelambert@niaid.nih.gov, satos@niaid.nih.gov, herrodja@gmail.com, yvette.robbins@nih.gov, carterd4@niaid.nih.gov, and patrick.duffy@nih.gov. Stomy Bin Shamamba Karhemere and Pati Pyana, Department of Parasitology, Institut National de Recherche Biomédicale (INRB), Kinshasa, Democratic Republic of Congo, E-mails: stomy_karhem@yahoo.fr and ppyana@yahoo.fr. Philippe Büscher, Department of Biomedical Sciences, Prince Leopold Institute of Tropical Medicine, Antwerp, Belgium, E-mail: pbuscher@itg.be.
References
- 1.Jaffe RI, Lowell GH, Gordon DM. Differences in susceptibility among mouse strains to infection with Plasmodium berghei (ANKA clone) sporozoites and its relationship to protection by gamma-irradiated sporozoites. Am J Trop Med Hyg. 1990;42:309–313. doi: 10.4269/ajtmh.1990.42.309. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay R, Conteh S, Li M, James ER, Epstein JE, Hoffman SL. The effects of radiation on the safety and protective efficacy of an attenuated Plasmodium yoelii sporozoite malaria vaccine. Vaccine. 2009;27:3675–3680. doi: 10.1016/j.vaccine.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Perignon JL, Van Marck E, Druilhe P. How reliable are models for malaria vaccine development? Lessons from irradiated sporozoite immunizations. J Postgrad Med. 2006;52:321–324. [PubMed] [Google Scholar]
- 4.Bray RS. Vaccination against Plasmodium falciparum: a negative result. Trans R Soc Trop Med Hyg. 1976;70:258. doi: 10.1016/0035-9203(76)90054-7. [DOI] [PubMed] [Google Scholar]
- 5.Edelman R, Hoffman SL, Davis JR, Beier M, Sztein MB, Losonsky G, Herrington DA, Eddy HA, Hollingdale MR, Gordon DM, Clyde DF. Long-term persistence of sterile immunity in a volunteer immunized with X-irradiated Plasmodium falciparum sporozoites. J Infect Dis. 1993;168:1066–1070. doi: 10.1093/infdis/168.4.1066. [DOI] [PubMed] [Google Scholar]
- 6.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, Nussenzweig M, Clyde RS, Edelman DR. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee S, Ngonseu E, Van Overmeir C, Correwyn A, Druilhe P, Wery M. Rodent malaria in the natural host–irradiated sporozoites of Plasmodium berghei induce liver-stage specific immune responses in the natural host Grammomys surdaster and protect immunized Grammomys against P. berghei sporozoite challenge. Afr J Med Med Sci. 2001;30((Suppl)):25–33. [PubMed] [Google Scholar]
- 8.Lecompte E, Granjon L, Peterhans JK, Denys C. Cytochrome b-based phylogeny of the Praomys group (Rodentia, Murinae): a new African radiation? C R Biol. 2002;325:827–840. doi: 10.1016/s1631-0691(02)01488-9. [DOI] [PubMed] [Google Scholar]
- 9.LUM Center Plasmodium berghei: Life Histories and Stabilates (Deep-Frozen Samples) of Isolates, Lines, and Clones Maintained at the University of Edinburgh. https://www.lumc.nl/sub/1040/att/811071156182556/811130228382556/908051142222557.pdf Available at. Accessed November 8, 2016.
- 10.Killick-Kendrick R. Parasitic protozoa of the blood of rodents: a revision of Plasmodium berghei. Parasitology. 1974;69:225–237. doi: 10.1017/s0031182000048071. [DOI] [PubMed] [Google Scholar]
- 11.Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, McCoy BN, Safronetz D, Sogoba N, Maiga O, Traore SF. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, west Africa, and the potential for human infection. PLoS Negl Trop Dis. 2012;6:e1924. doi: 10.1371/journal.pntd.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieterlen F. Genus Grammomys thicket rats. In: Happold DCD, editor. Mammals of Africa. Vol. III. London, United Kingdom: Bloomsbury Publishing; 2013. pp. 404–405. [Google Scholar]
- 13.Happold DCD. Grammomys dolichurus woodland thicket rat (Common Grammomys) In: Happold DCD, editor. Mammals of Africa. Vol. III. London, United Kingdom: Bloomsbury Publishing; 2013. pp. 410–411. [Google Scholar]
- 14.Matuschewski K, Hafalla JC, Borrmann S, Friesen J. Arrested Plasmodium liver stages as experimental anti-malaria vaccines. Hum Vaccin. 2011;7((Suppl)):16–21. doi: 10.4161/hv.7.0.14557. [DOI] [PubMed] [Google Scholar]
- 15.Siu E, Ploss A. Modeling malaria in humanized mice: opportunities and challenges. Ann N Y Acad Sci. 2015;1342:29–36. doi: 10.1111/nyas.12618. [DOI] [PubMed] [Google Scholar]
- 16.Waters H. Better animal models needed for malaria vaccine development, experts say. 2011. http://blogs.nature.com/spoonful/2011/10/better_animal_models_needed_fo_1.htm Available at. Accessed.
- 17.Perkins SL. Molecular systematics of the three mitochondrial protein-coding genes of malaria parasites: corroborative and new evidence for the origins of human malaria. Mitochondrial DNA. 2008;19:471–478. doi: 10.1080/19401730802570926. [DOI] [PubMed] [Google Scholar]
- 18.De Niz M, Ullrich AK, Heiber A, Blancke Soares A, Pick C, Lyck R, Keller D, Kaiser G, Prado M, Flemming S, Del Portillo H, Janse CJ, Heussler V, Spielmann T. The machinery underlying malaria parasite virulence is conserved between rodent and human malaria parasites. Nat Commun. 2016;7:11659. doi: 10.1038/ncomms11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larremore DB, Sundararaman SA, Liu W, Proto WR, Clauset A, Loy DE, Speede S, Plenderleith LJ, Sharp PM, Hahn BH, Rayner JC, Buckee CO. Ape parasite origins of human malaria virulence genes. Nat Commun. 2015;6:8368. doi: 10.1038/ncomms9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins SL, Sarkar IN, Carter R. The phylogeny of rodent malaria parasites: simultaneous analysis across three genomes. Infect Genet Evol. 2007;7:74–83. doi: 10.1016/j.meegid.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Perkins SL, Schall JJ. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Yoeli M, Alger N, Most H. Tree rat, Thamnomys surdaster surdaster, in laboratory research. Science. 1963;142:1585–1586. doi: 10.1126/science.142.3599.1585. [DOI] [PubMed] [Google Scholar]
- 23.Yoeli M, Most H. A study of Plasmodium berghei in Thamnomys surdaster, and in other experimental hosts. Am J Trop Med Hyg. 1964;13:659–663. doi: 10.4269/ajtmh.1964.13.659. [DOI] [PubMed] [Google Scholar]
- 24.Yoeli M. Slow maturing primary exo-erythrocytic schizonts of Plasmodium berghei in an experimentally infected tree-rat (Thamnomys surdaster) Trans R Soc Trop Med Hyg. 1974;68:302–305. doi: 10.1016/0035-9203(74)90037-6. [DOI] [PubMed] [Google Scholar]
- 25.Buscher P, Bin Shamamba SK, Ngoyi DM, Pyana P, Baelmans R, Magnus E, Van Overmeir C. Susceptibility of Grammomys surdaster thicket rats to Trypanosoma brucei gambiense infection. Trop Med Int Health. 2005;10:850–855. doi: 10.1111/j.1365-3156.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 26.Musser GG, Carlteon MD. Superfamily Muroidea. In: Wilson D, Reeder D, editors. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd edition. Baltimore, MD: Johns Hopkins University Press; 2005. pp. 894–1531. [Google Scholar]
- 27.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 28.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL. the VRC 312 Study Team Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.