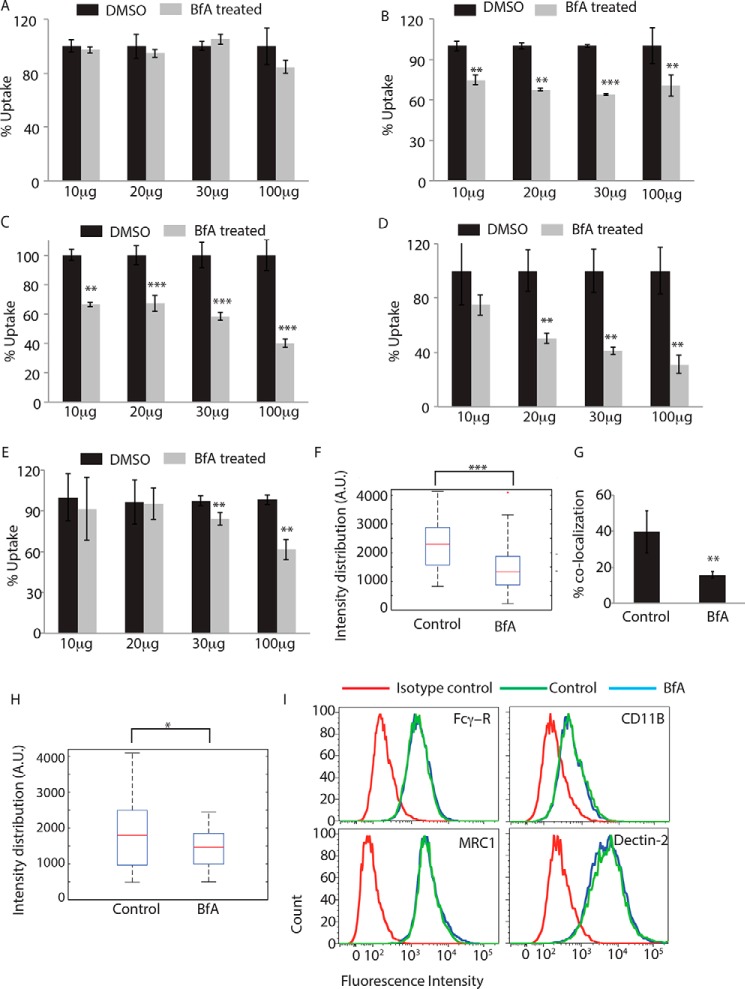
FIGURE 4.
Role of Golgi apparatus during phagocytosis. THP-1 macrophages were incubated with human IgG-coated FITC-labeled latex beads (A), serum-coated FITC-labeled latex beads (B), complement-deactivated serum-coated latex beads (C), uncoated latex beads (D), or E. coli (E) for 30 min in the absence or presence of brefeldin A at concentrations mentioned. BfA treatment was given 30 min prior to the addition of the beads or E. coli. Samples were acquired in a flow cytometer to measure uptake. Plots represent data from an average of three independent experiments. Error bars represent S.D. (**, p value <0.01; ***, p value <0.005). F, THP-1 macrophages were allowed to uptake serum-coated FITC-labeled latex beads for 30 min in the presence or absence of BfA. Samples were fixed and stained for mannosidase-II using anti-MAN2A antibody followed by Alexa Fluor 405 secondary antibody. Confocal images acquired were analyzed using 3D module in Imaris 7.2 software to calculate mannosidase-II intensity distribution over the latex beads (***, p value <0.001). G, GFP expressing E. coli was incubated with THP-1 macrophages for 30 min in the presence or absence of BfA. Samples were fixed and stained for mannosidase-II using anti-MAN2A antibody followed by Alexa Fluor 405 secondary antibody. Confocal images were analyzed using Imaris software to calculate percent co-localization. H, THP-1 macrophages were allowed to uptake IgG-coated FITC-labeled latex beads for 30 min in the presence or absence of BfA. Samples were fixed and stained for mannosidase-II using anti-MAN2A antibody followed by Alexa Fluor 405 secondary antibody. Confocal images acquired were analyzed using 3D module in Imaris software to calculate mannosidase-II intensity distribution over the latex beads. Co-localization (*, p value <0.05). I, U937-derived macrophages were incubated with 20 μg/ml brefeldin A for 60 min, fixed, and stained for receptors mentioned. Cells were acquired by flow cytometry and overlays analyzed using FlowJo.