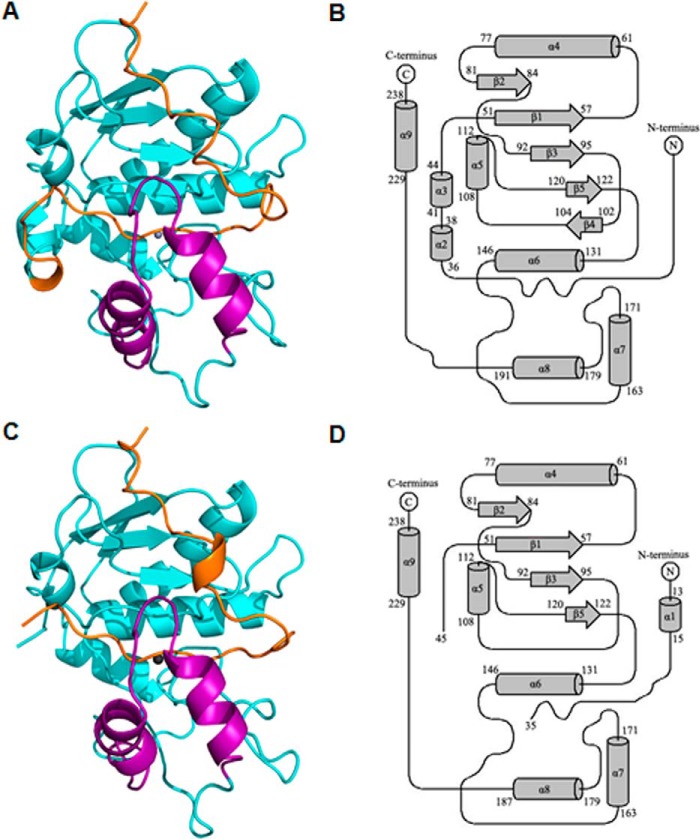
FIGURE 2.
Overall structure of pro-myroilysin. A, overall structure of pro-myroilysin, solved at 1.89 Å. The pro-peptide is colored orange; the polypeptide chain composed of residues 160–193 is purple; the zinc ion is gray; other parts are cyan. B, topology of the pro-myroilysin structure solved at 1.89 Å. The N-terminal domain is formed by five helices (α3–6 and α9) and four β-strands (β1–5). The C-terminal domain is formed by two helices (α7–8) and coils. C, overall structure of pro-myroilysin solved at 1.6 Å. The pro-peptide is orange; the polypeptide chain composed of residues 160–193 is purple; the zinc ion is gray; other parts are cyan. D, topology of the pro-myroilysin structure solved at 1.6 Å. The N-terminal domain is formed from four helices (α4–6 and α9) and four β-strands (β1–3 and β5). The C-terminal domain is formed from two helices (α7–8) and coils. The diagrams were drawn using TopDraw.