FIGURE 8.
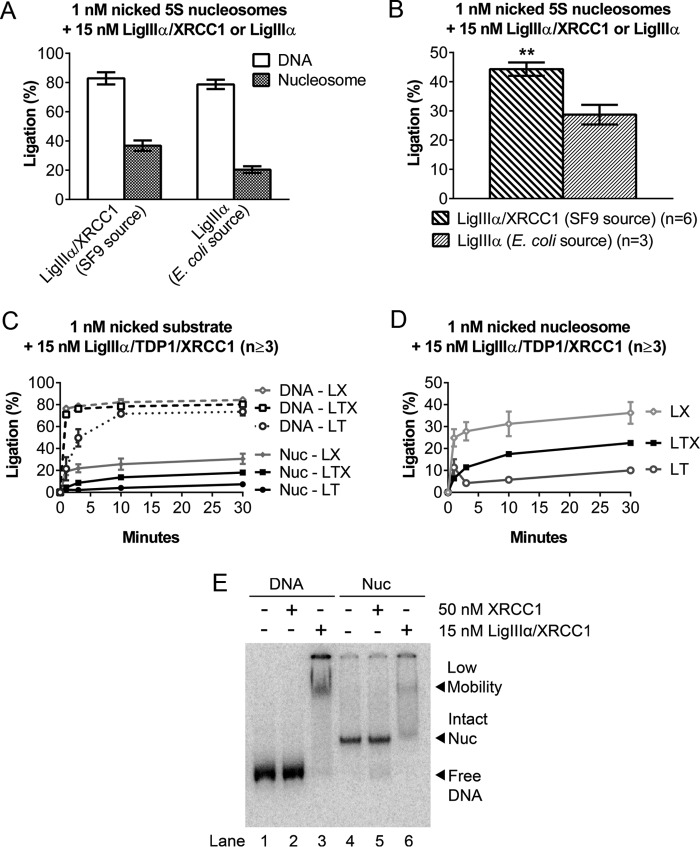
XRCC1 promotes ligation of nicks by LigIIIα. A, DNA nick-containing, 5S rDNA-based nucleosomes were incubated at 37 °C for 30 min with either 15 nm Sf9-expressed LigIIIα-XRCC1 or an equivalent amount of E. coli-expressed LigIIIα (using concentrations specified by the supplier), and the extent of ligation was measured as before. B, comparison of nucleosome ligation efficiency between LigIIIα-XRCC1 and LigIIIα. The values shown are based on data from A after normalizing to the extent of ligation obtained in reactions with the corresponding naked DNAs. In the absence of XRCC1, the extent of ligation was ∼⅔ that observed with LigIIIα-XRCC1. C, time course of 15 nm Sf9-expressed LigIIIα-TDP1 and LigIIIα-TDP1-XRCC1 incubated with 1 nm nick-containing DNA or nucleosomes (Nuc) under conditions identical to Figs. 1D and A. Data from Fig. 1D are included to facilitate comparisons. LT, LigIIIα-TDP1; LX, LigIIIα-XRCC1; LTX, LigIIIα-TDP1-XRCC1. D, ligation products from reactions with nucleosomes in C were recalculated as a fraction of naked DNA ligation to adjust for differing naked DNA ligation efficiencies. Note that ligation efficiencies were markedly higher in complexes containing XRCC1. E, in gel shift experiments conducted as in Fig. 3, E. coli-purified recombinant XRCC1 did not detectably alter the mobility of nucleosomes containing a single nucleotide gap DNA/nucleosomes. This result suggests that nucleosome binding by LigIIIα-XRCC1 is driven primarily by DNA ligase IIIα. Error bars represent standard deviations; ** = p < 0.01.